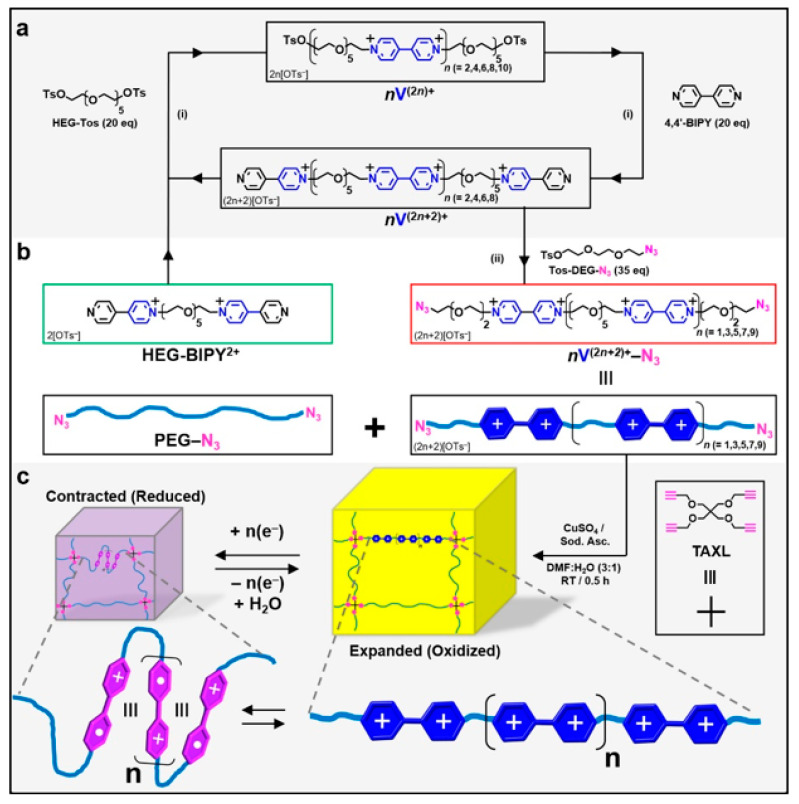
Figure 9.
(a) An iterative synthesis was used to prepare each even-numbered (n = 2, 4, 6, 8, and 10) oligoviologen by (i) alternating between the excessive addition (20 equiv) of tosyl end-capped hexaethylene glycol (HEG-Tos) and 4,4′-bipyridine (BIPY) in MeCN at 130 °C for 12–16 h in a closed reaction vessel. (b) The synthetic cycle begins (green box) with a BIPY end-capped HEG (HEG-BIPY2+), and the oligomer is grown iteratively, with only intermittent precipitations in MeCN:PhMe, followed by centrifugation in order to isolate each product. At any point in the cycle, the BIPY end-capped precursor can be removed and (ii) functionalized with terminal azide groups (red box) through the excessive addition (35 equiv, MeCN, 130 °C, 20 h) of a tosylated diethylene glycol possessing one azide at its terminus (Tos-DEG-N3). (c) Synthesis of the click-based hydrogel involves 2 equiv of bis-azide-terminated linkers, where 95 mol % of the 2 equiv is composed of polyethylene glycol (PEG-N3) and 5 mol % consists of the oligoviologen (nV(2n) +-N3), to 1 equiv of the tetra-alkyne cross-linker (TAXL). Published in [176]. Copyright© 2017 American Chemical Society.