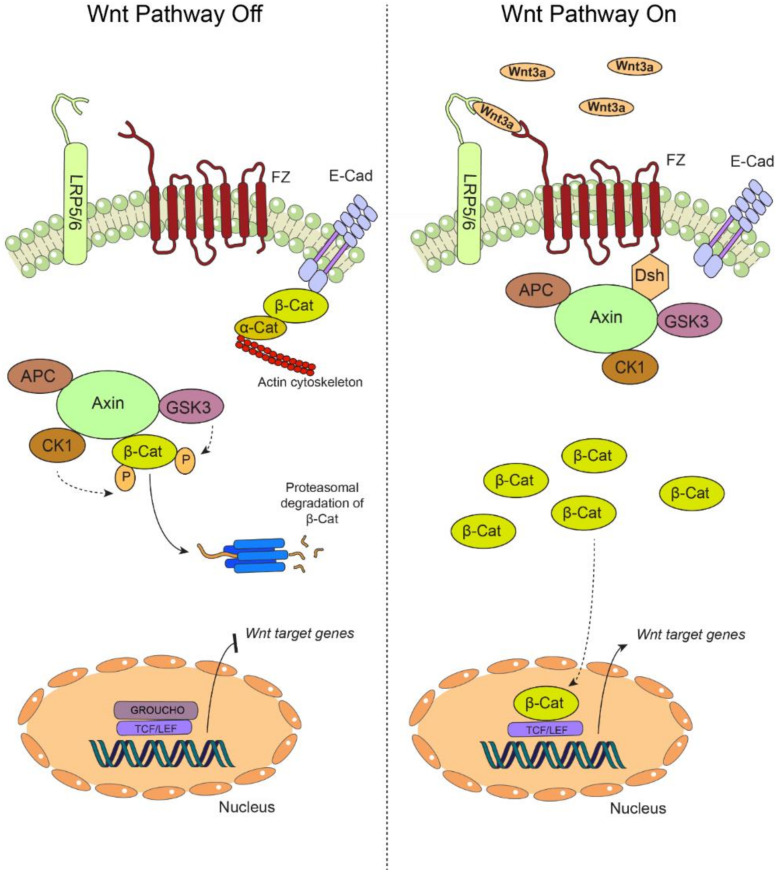
Figure 1.
The canonical Wnt pathway. (Left) In the absence of extracellular Wnt ligands, the transmembrane receptors Frizzled (FZ) and the coreceptors LDL (low-density lipoprotein) receptor related protein 5/6 (LRP 5/6) are unable to associate at the plasma membrane, yielding an “off” state of the pathway. During this off state, β-catenin (β-Cat) is mainly found at cell-cell adhesion complexes, bridging together the intercellular adhesion molecule E-Cadherin (E-cad) and the actin cytoskeleton via interaction with α-catenin (α-Cat). In the cytoplasm, β-catenin is rapidly targeted for proteasomal degradation by the so-called “destruction complex”, which is composed by adenomatous polyposis coli (APC), axin, casein kinase 1 (CK1) and glycogen synthase kinase 3β (GSK3β). The targeting of β-catenin for degradation is based on sequential phosphorylation by CK1 and GSK3β. In these conditions, β-catenin cannot translocate to the nucleus, and the transcription of the target genes is repressed by GROUCHO, which is bound to the TCF/LEF promoters. (Right) Secreted Wnt ligands, such as Wnt3a, are recognized by both FZ and LRP5/6, switching “on” the pathway. The destruction complex is then recruited to the plasma membrane via interaction with the FZ receptor, allowing the cytoplasmic accumulation of β-catenin, which is now available for translocation to the nucleus, where it binds the TCF/LEF promoter by displacing GROUCHO, allowing the transcription of Wnt target genes. Particularly, in oral carcinogenesis, this pathway is “switched on” by the increased secretion of Wnt3a, stabilization of β-catenin and the expression of target genes such as cyclin D1 and survivin (see main text for details).