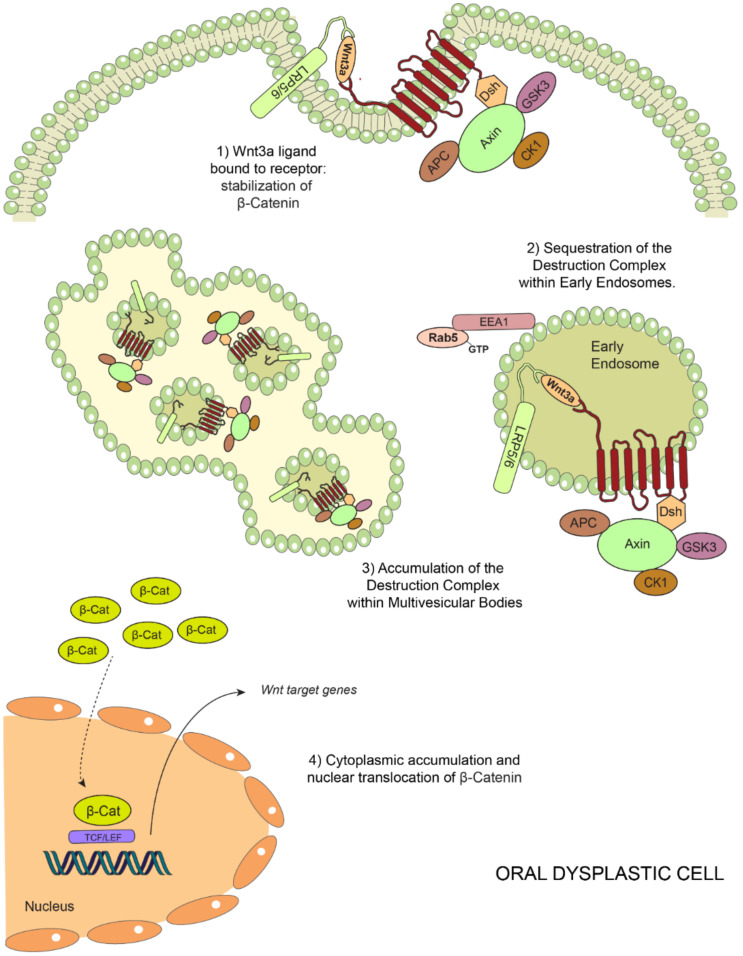
Figure 5.
Model of endosomal sequestration of the destruction complex in oral dysplasia. Binding of Wnt3a to Frizzled (FZ) and LRP5/6 leads to the recruitment of the destruction complex to the FZ-LRP5/6 receptor complex at the plasma membrane. This is followed by a decreased proteasomal degradation of β-catenin (1). Ligand binding and subsequent posttranslational modifications (not described in this scheme) lead to endocytosis of this supramolecular complex, also known as the “Wnt signalosome complex”, and subsequent trafficking en route to early endosomes and multivesicular bodies in a Rab5-dependent manner (2). In oral dysplasia, high Wnt3a levels and increased Rab5 activity lead to the enhanced sequestration of the destruction complex within EEA1-positive early endosomes. Consequently, higher levels of components of the destruction complex are detected in multivesicular bodies (3). These events ultimately lead to a more robust stabilization of β-catenin in the cytoplasm and a consequent nuclear translocation in order to bind TCF/LEF factors, activating the transcription of the target genes (4).