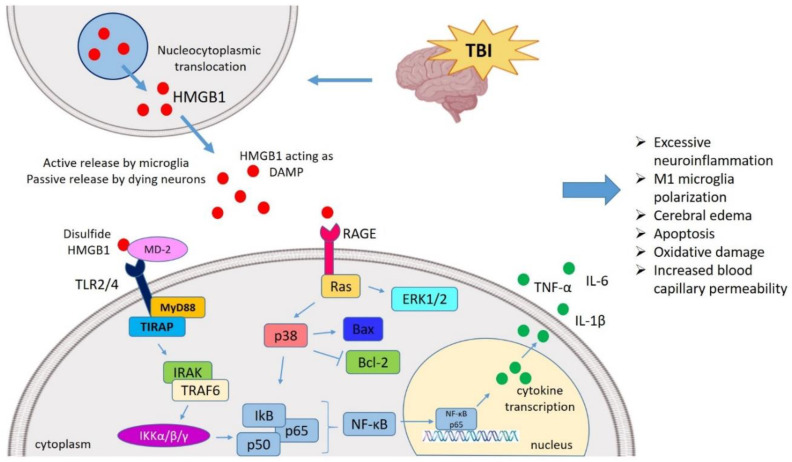
Figure 1.
HMGB1 mediated neuroinflammatory response in TBI. TBI induces nucleocytoplasmic translocation of HMGB1 resulting into the release of HMGB1 in extracellular milieu. The extracellular HMGB1 may be partially oxidized at the two cysteine residues generating the disulfide form of HMGB1. The disulfide HMGB1 further binds to its prominent receptor system such as TLR4 and RAGE which in turn interacts with MD-2 and initiates the MyD88 dependent pathway. It also binds to Ras to initiate the ERK pathway, respectively. HMGB1-TLR4 axis can activate NF-κB signaling both directly and through TRAF6. These pathways ultimately interact with the NF-κB lead to the generation of neuroinflammatory response by producing several pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6). In this way, HMGB1 might mediate the TBI-induced secondary injury where HMGB1 is understood to amplify vicious neuroinflammation, M1 polarization, apoptosis, oxidative damage, cerebral edema, increased BBB permeability. TBI, Traumatic brain injury; HMGB1, High mobility group box 1; TLR4, Toll-like receptor 4; MD-2, Myeloid differentiation factor-2; MyD88, Myeloid differentiation response protein 88; ERK, Extracellular signal-related kinase; NF-κB, Nuclear factor κ light chain enhancer of activated B cells; TNF-α, Tumor necrosis factor-α; IL, Interleukin; BBB, Blood–brain barrier; TRAF6, TNF receptor-associated factor 6.