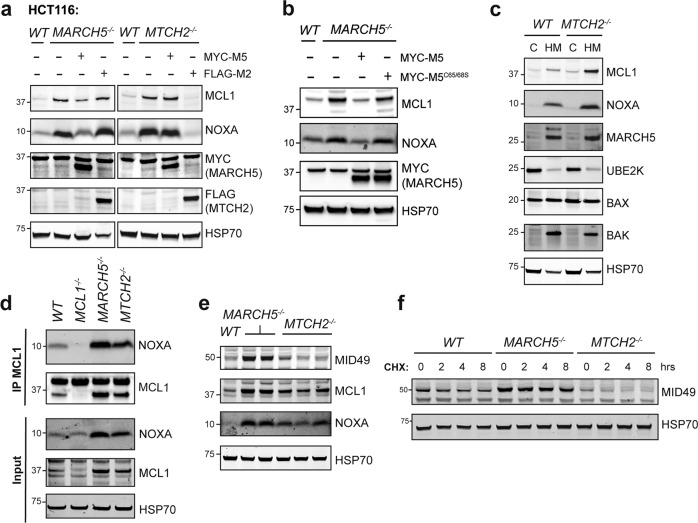
Fig. 7. MTCH2 and MARCH5 jointly control turnover of the MCL1:NOXA complex.
a MARCH5 and MTCH2 require each other to regulate MCL1 degradation by NOXA. WT, MARCH5−/−, or MTCH2−/− HCT116 cells were engineered to stably express either MYC-MARCH5 (MYC-M5) or FLAG-MTCH2 (FLAG-M2). MYC-MARCH5 restored NOXA-driven MCL1 turnover in MARCH5−/− but not MTCH2−/− cells, while FLAG-MTCH2 did so in MTCH2−/− but not MARCH5−/− cells. b The E3 ubiquitin ligase function of MARCH5 is critical for regulating MCL1 degradation. MARCH5−/− HCT116 cells were engineered to express either WT MARCH5 or a ligase-defective mutant (MARCH5C65/68S). While WT MARCH5 restored NOXA-driven MCL1 turnover, MARCH5C65/68S did not. c MTCH2 is not required for the heavy membrane association of key proteins required for NOXA-driven MCL1 turnover. Protein lysates were prepared from cytosol (C) and heavy membrane (HM) fractions derived from WT and MTCH2−/− HCT116 cells. d MCL1 was immunoprecipitated from HCT116 cells (WT, MCL1−/−, MARCH5−/−, and MTCH2−/−) cultured with proteasome inhibitor MG132 (10 μM for 8 h). NOXA continued to interact with MCL1 even when MTCH2 was absent. e The steady-state expression level of MID49 was elevated in MARCH5−/− but not MTCH2−/− HCT116 cells. f The rate MID49 protein turnover was reduced in MARCH5−/− but not MTCH2−/− HCT116 cells. Cells were cultured with the protein synthesis inhibitor cycloheximide (50 μg/mL; CHX) for up to 8 h.