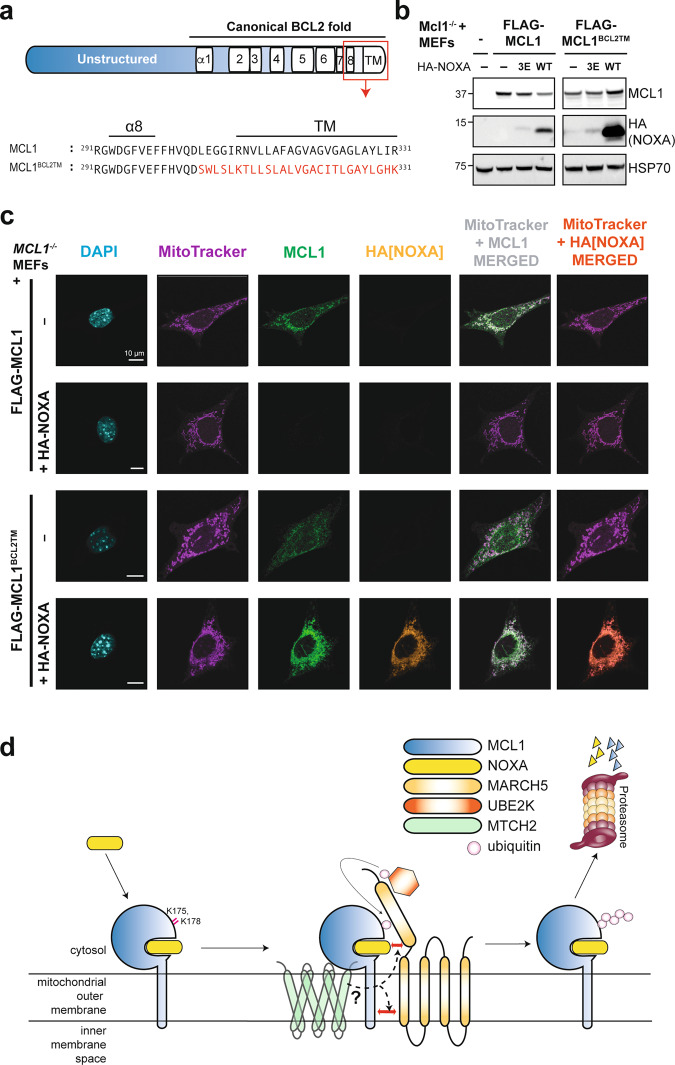
Fig. 8. Elements of the MCL1 transmembrane domain are necessary for its turnover by MARCH5 and MTCH2.
a Schematic highlighting the MCL1 transmembrane domain and a mutant in which this region of MCL1 was replaced by corresponding sequence from BCL2. b The MCL1 transmembrane domain is critical for NOXA to trigger MCL1 degradation. Mcl1−/− MEFs were engineered to express FLAG-MCL1 or FLAG-MCL1BCL2TM along with HA-NOXA or HA-NOXA3E. c Mcl1−/− MEFs engineered to express FLAG-MCL1 or FLAG-MCL1BCL2TM with or without HA-NOXA were imaged by confocal immunofluorescence microscopy. MCL1 (green) and NOXA (orange) were revealed by MCL1 and HA immunofluorescence, whilst DAPI (blue) and MitoTracker (purple) provided counterstain for the nucleus and mitochondrial network respectively. When co-expressed, FLAG-MCL1BCL2TM and HA-NOXA were both present at high levels on the mitochondrial network (merged channels) indicating that degradation of the MCL1:NOXA complex by MARCH5/MTCH2 requires the MCL1 transmembrane domain. Scale bars: 10 μm. Images are representative of at least 10 fields of view captured for each condition. Equal laser intensities and thresholds were used in capturing and representing the MCL1 and HA immunofluorescence signals to faithfully represent their relative levels between samples. d Model highlighting key aspects of MARCH5-meditated turnover of MCL1. MARCH5 degrades MCL1 specifically when it is engaged by NOXA, suggesting that NOXA may contribute to the motif through which MARCH5 recognizes MCL1 as a substrate. Our findings also indicate that the integral mitochondrial outer membrane protein MTCH2, the transmembrane domain of MCL1, and key lysine residues on MCL1 are all critical elements of this mechanism.