Fig. 14.3.
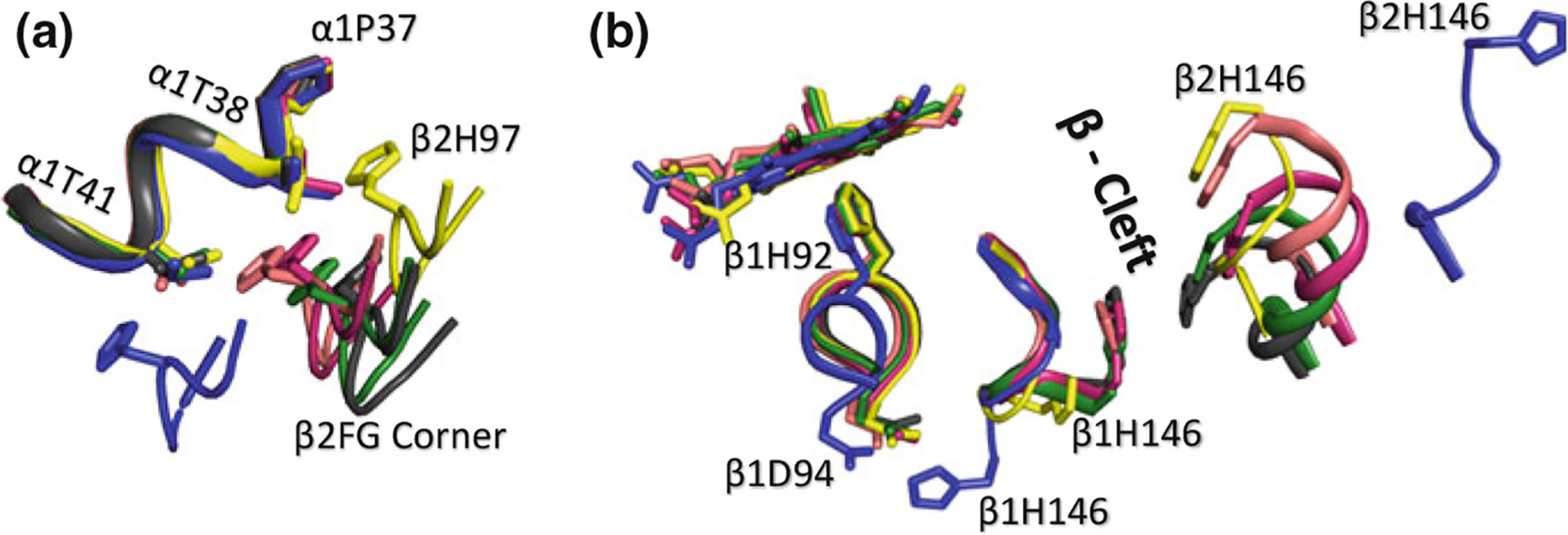
Superposed structures of T (blue), R (magenta), R3 (yellow), RR2 (green), R2 (black), and RR3 (salmon) on their α1β1 dimers. a Transitions between the different states lead to significant changes (sliding motion) at the α1β2 dimer interface switch regions. b Transitions from the T state to the relaxed states breaks a T state stabilizing salt-bridge interaction between βAsp94 and βHis146. In the R2 and RR2 structures β1His146 makes close contact with β2His146, while in the other relaxed structures, βHis146 becomes highly disordered. There is also a significant size decrease in the β-cleft of the relaxed structures compared to the T structure
