Fig. 14.9.
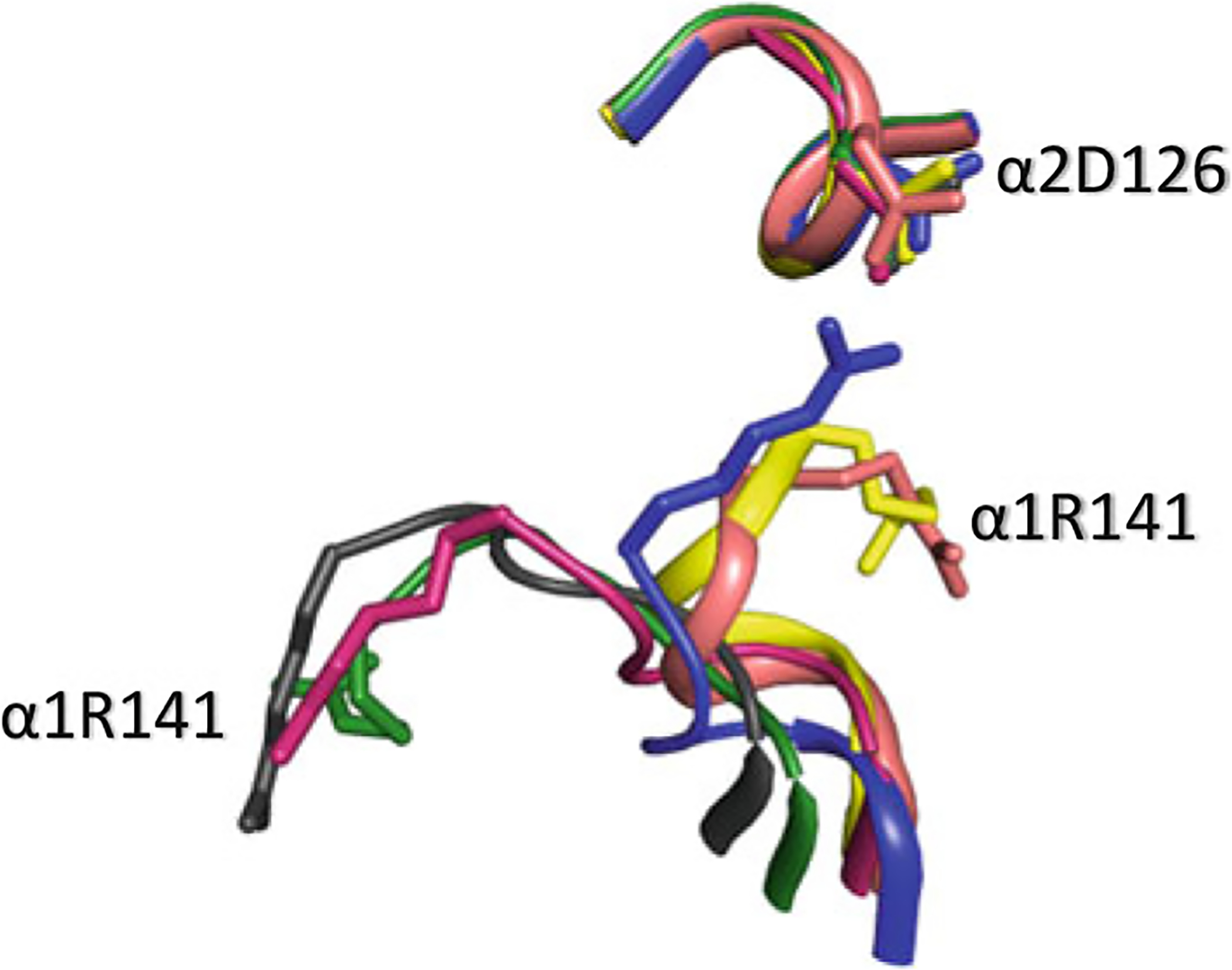
Superposed structures of T (blue), R (magenta), R3 (yellow), RR2 (green), R2 (black), and RR3 (salmon) on their α1β1 dimers. αArg141 participates in inter-subunit salt-bridge interaction with αAsp126 (as well as αLys127—not shown) in deoxygenated Hb stabilizing the low-affinity T state and facilitating O2 release. At higher pH, this interaction is broken increasing the mobility of αArg141, which facilitates the T → R transition, and increase Hb oxygen affinity
