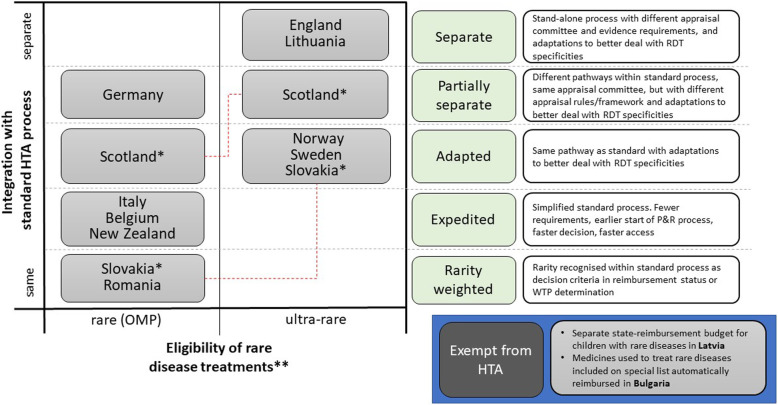
Fig. 2.
Classification of supplemental processes by level of integration and applicability to rare versus ultra-rare. This figure provides an overview of the study countries that have supplemental processes for the routine use of rare and/or ultra-rare disease treatments in a defined patient population within a health service. * Scotland and Slovakia have two different supplemental pathways for rare and ultra-rare disease treatments respectively, which are differentiated here. ** Rare disease treatment with orphan designation from European Medicines Agency (“Orphan Medicinal Product”, OMP); ultra-rare disease treatment defined by individual country definitions, often alongside other criteria. RDT: rare disease treatment; OMP: orphan medicinal product; P&R: pricing and reimbursement process