Figure 4.
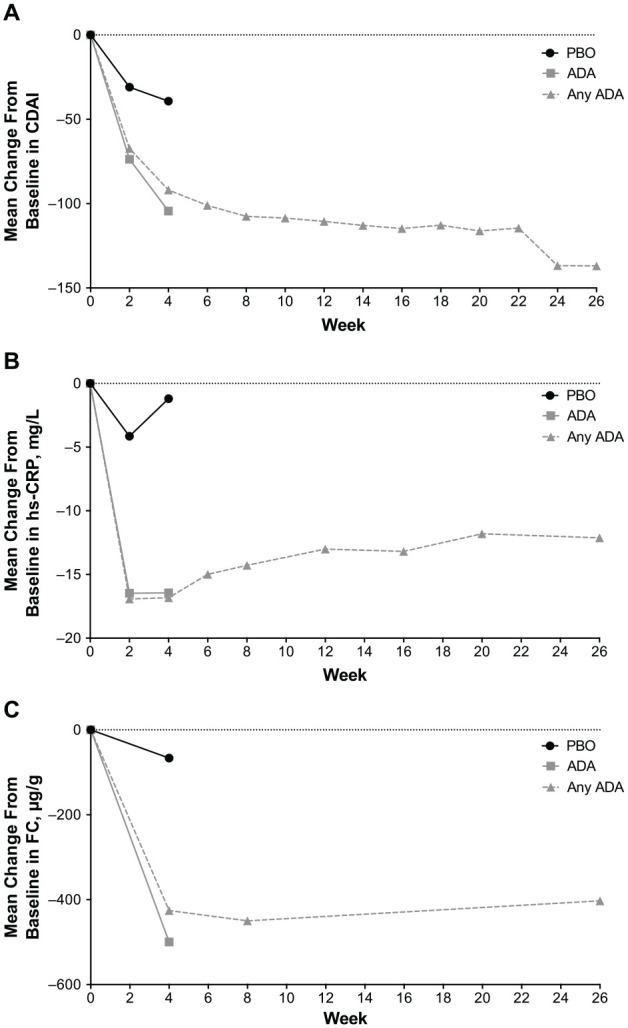
Mean change from baseline in (A) CDAI (LOCF), (B) hs-CRP (LOCF), and (C) FC (LOCF). For the ADA and PBO treatment groups, change from baseline at weeks 2 and 4 was analyzed for the intent-to-treat population. For the any-adalimumab set (any ADA), baseline and visits were relative to the first dose of ADA. FC was collected at baseline and weeks 4, 8, and 26.
ADA, adalimumab; CDAI, Crohn’s disease activity index; FC, fecal calprotectin; hs-CRP, high-sensitivity C-reactive protein; LOCF, last observation carried forward; PBO, placebo.
