Abstract
Objectives
In this study, a new immunoassay for the simultaneous determination of pepsinogen I (PGI) and pepsinogen II (PGII) in serum based on element labeling strategy coupled with inductively coupled plasma mass spectrometry (ICP‐MS) detection was proposed.
Methods
The sandwich‐type immunoassay was used to simultaneously detect PGI and PGII in serum. PGI and PGII were captured by anti‐PGI and anti‐PGII antibody immobilized on the magnetic beads and then banded with Eu3+ labeled anti‐PGI detection antibody and Sm3+ labeled anti‐PGII detection antibody, followed by ICP‐MS detection.
Results
The linear correlation coefficient (R 2) of PGI and PGII standard curves was .9938 and .9911, with the dynamic range of 0‐200 ng/mL and 0‐60 ng/mL, respectively. The limit of detection for PGI and PGII was 1.8 ng/mL and 0.3 ng/mL, respectively. The average recovery was 101.41% ± 6.74% for PGI and 101.47% ± 4.20% for PGII. Good correlations were obtained between the proposed method and CLIA (r = .9588 for PGI, r = .9853 for PGII).
Conclusions
We established a mass spectrometry‐based immunoassay for the simultaneous detection of PGI and PGII in a single analysis. The element tagged immunoassay coupled with ICP‐MS detection has high sensitivity, accuracy, and specificity in clinical serum sample analysis.
Keywords: element tagged immunoassay, ICP‐MS, multiplex immunoassay, pepsinogen I, pepsinogen II
1. INTRODUCTION
Gastric cancer is one of the most common cancer and leading cause of cancer‐related death in the world.1, 2 Over the past decades, the pepsinogen (PG) test has been widely used to evaluate the gastric function and gastric cancer screening.3, 4 In blood, pepsinogens secreted by gastric mucosa are divided into pepsinogen I (PGI) and pepsinogen II (PGII), which is closely related to the classification of gastric disease.5, 6 PGI, PGII, and the ratio of PGI/PGII are correlated with atrophic gastritis, and a low PGI level (≤70 ng/mL) and PGI/PGII ratio (≤3) are frequently found in gastric cancer.7, 8 Therefore, it is great significant to simultaneously detect PGI and PGII for early diagnosis and treatment of gastric cancer.
Over the past decades, several methods have been reported in determination of PGI and PGII, including enzyme‐linked immunosorbent assay (ELISA),9 time‐resolved fluoroimmunoassay (TRFIA),10 chemiluminescent immunoassay (CLIA),11 and electrochemiluminescence immunoassay (ECLA).12 However, these traditional methods, which only measure one target in a single analysis, are often limited by simultaneous multiplex analysis owing to limited number of labels or signal overlapping. Therefore, it is crucial to develop a sensitive, rapid, and multiplex assay for measuring PGI and PGII.
Element tagged immunoassay coupled with inductively coupled plasma mass spectrometry (ICP‐MS) detection is a powerful technique for simultaneous determination of multiplex biomolecules.13, 14, 15 In this technique, the rare earth elements (REEs) are used as the labels for multiplex labeling, following multiplex detection by ICP‐MS. Since the pioneer work was proposed by our group,16 this method has been widely used in measuring clinical biomarkers and single‐cell analysis.17, 18, 19, 20 Compared with the traditional labels, such as radioisotope, fluorescent dye, and enzyme, the REEs can be sensitively and simultaneously measured without signal overlapping and low blank signal. Therefore, it is very valuable for the element tagged immunoassay combined with ICP‐MS detection to measure multiple biomarkers in the clinical laboratories.
In this study, we report an element tagged immunoassay for simultaneous quantification of PGI and PGII in human serum samples, which take advantage of magnetic beads for fast capture and separation, stable isotope element of samarium (Sm) and europium (Eu) for dual‐labeling, and ICP‐MS for multiplex readout. The combination of ICP‐MS analytical device and stable isotope element tags highly improves the multiplex capability in a single analysis, which provides a promising tool for the early diagnosis of gastric cancer.
2. MATERIALS AND METHODS
2.1. Reagents and chemicals
The Eu3+‐labeled monoclonal anti‐PGI detection antibody (Medix, 8016#) and Sm3+‐labeled monoclonal anti‐PGII detection antibody (Medix, 8102#) were purchased from Wuxi Jiangyuan Industrial Technology and Trade Corporation. The monoclonal anti‐PGI capture antibody (Medix, 8003#), monoclonal anti‐PGII capture antibody (Medix, 8101#), and PGI and PGII antigen were also purchased from the same company. All the buffers used in the experiment were obtained from this company. The amino magnetic beads (2.0 μm) were purchased from Suzhou Beaver Biomedical Engineering Co., Ltd. The 1‐ethyl‐3‐(3‐dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N‐hydroxysulfosuccinimide (NHS) were purchased from Sigma Chemical Co. The ultrapure water (18.2 MΩ cm) obtained from a Milli‐Q water purification system (Millipore Milford) was used throughout the experiment. All reagents were at least analytical or higher grade.
2.2. Instruments
An iCAP Q ICP‐MS (Thermo Fisher Scientific) was used in the experiments. The instrument was configured with a quartz cyclonic spray chamber and a concentric, PFA low flow nebulizer. The peristaltic pump tubing was orange/green tubing for carrier (sample) and orange/yellow tubing for internal standard. The rhenium (Re, 5 ppb) was chosen as the internal standard element for detection. The ICP‐MS was used in STD mode, and instrument settings were optimized prior to analysis. Typically, the peristaltic pump speed was 40 rpm, RF power was 1550 W, the cool gas flow was 14 L/min, auxiliary gas flow was 0.8 L/min, and nebulizer gas flow was 0.92 L/min.
2.3. Clinical samples and comparison method
A total number of 103 serum samples from routine analysis of 103 subjects (52 males and 51 females, ages 24‐85 years), who were referred to the PLA General Hospital, were collected for the study. The PGI and PGII values of the collected samples were measured by CLIA (Abbott Diagnostic). These samples were stored at −20°C before analysis. The study was approved by the Ethical Committee of the PLA General Hospital (Number: S2018‐007‐01).
2.4. Preparation of the antibody‐magnetic beads conjugates
The antibody‐magnetic beads conjugates were synthesized according to the previous method.21 Briefly, the magnetic beads (100 μL, 10 mg/mL) were firstly activated by the mixture solution of NHS (200 μL, 50 mg/mL) and EDC (200 μL, 10 mg/mL) in the MES solution (0.1 M, pH 5.0) for 2 hours with gentle shaking at 25°C. Then, 1 mg anti‐PGI/PGII antibody was added to the activated magnetic beads in PBS solution (0.01 M, pH 7.4). The reaction was allowed to process for 18 hours with gentle shaking at 25°C. After washing four times by PBST solution, the antibody‐magnetic beads conjugates were resuspended in Tris‐HCl (0.05 M, pH 7.4) and stored at −4°C.
2.5. Preparation of PGI and PGII standards
Pure reference PGI and PGII standards were mixed in equivalent volume in the Tris‐HCl buffer (0.05 M, pH 7.4, 0.5% BSA) at the final concentrations of 0, 3, 6, 13, 25, 50, 100, 200 ng/mL for PGI standards and 0, 1, 2, 4, 8, 15, 30, 60 ng/mL for PGII standards.
2.6. Assay protocol
As shown in Figure 1, the proposed immunoassay for simultaneous quantification of PGI and PGII was sandwich immunoassay format. In each well, 50 μL magnetic beads anti‐PGI/PGII antibody were added and mixed with 50 μL of standards or samples. The tube was incubated for 10 minutes with continuous horizontal shaking at 37°C. After washing two times, 100 μL of Eu3+ labeled anti‐PGI detection antibody and Sm3+ labeled anti‐PGII detection antibody was added into the tube and incubated for 10 minutes with continuous horizontal shaking at 37°C. After washing three times, 100 μL/well of HNO3 solution (1%, v/v) was added, and the tube was incubated for 1 minute with gentle shaking. Finally, 100 μL of nitric acid supernatant was introduced into ICP‐MS, and the signal of Eu3+ and Sm3+ was simultaneously obtained.
Figure 1.
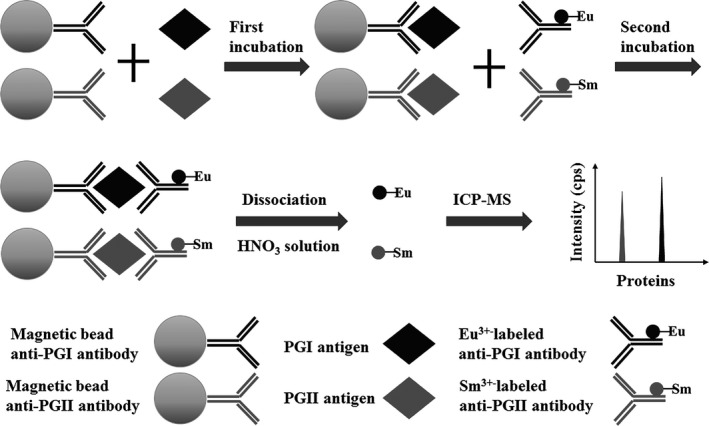
Illustration of the proposed method for pepsinogen I (PGI) and pepsinogen II detection principle
2.7. Standard curves and sensitivity assays
The standard curves of the assay were evaluated by a serial standard dilutions of PGI (0, 3, 6, 13, 25, 50, 100, 200 ng/mL) and PGII (0, 1, 2, 4, 8, 15, 30, 60 ng/mL). The limit of detection (LOD) was defined as the concentrations corresponding to the mean of 21 independent measurements of the zero standard plus three standard deviations (SDs). The sensitivity was determined as the lowest concentration.
2.8. Recovery assays
The recovery of the assay was performed by adding different concentrations of PGI and PGII standards to clinical serum samples. The recovery was calculated as: Recovery (%) = (measured concentration – original concentration)/spiked concentration × 100%.
2.9. Precision assays
The precision of the assay was performed by measuring the low and high concentrations of clinical samples. The samples were measured four runs daily at a time over a time period of 5 working days. The intra‐assay variations were obtained from 8 independent experiments, and the inter‐assay variations were obtained from 20 independent experiments.
2.10. Interference and specificity assays
The interference experiment was performed by measuring clinical serum samples with different interfering substance (bilirubin, triglyceride, and hemoglobin). The interference was expressed as bias. The equation was as follows: bias (%) = (measured concentration − original concentration)/original concentration × 100%. Furthermore, the investigation of antibody cross‐reactivity was performed, which was used to evaluate the specificity.
2.11. Statistical analysis
All the data analysis was performed with Origin 8.5 and GraphPad Prism software. Quantitative data were presented as mean ± SD. The standard curves were obtained by plotting the cps (y) against the sample concentration (x) using origin 8.5. Comparisons between quantitative data obtained from both methods were performed using paired t test and correlation analysis. Values of P < .05 were considered statistically significant.
3. RESULTS
3.1. Immunoassay optimization
The optimization of capture and detection antibody was firstly performed. The labeled antibodies from the reaction step were diluted 1:25, 1:50, 1:100, 1:200, 1:400, and 1:800, and the magnetic beads‐capture antibodies were 12.5, 25, 50, and 100 μL for each well. We simultaneously tested the PGI standards (0, 200 ng/mL) and PGII standards (0, 50 ng/mL). The background signal barely increased as the Eu/Sm‐labeled antibodies concentration increased, and the dilution with the highest signal that appeared first was selected. Therefore, we obtained the optimal dilutions of 1:100 for Eu‐labeled PGI antibodies (Figure 2A) and 1:200 Sm‐labeled PGII antibodies (Figure 2B). The magnetic beads‐capture antibodies for PGI and PGII were 50 μL (Figure 2C and D).
Figure 2.
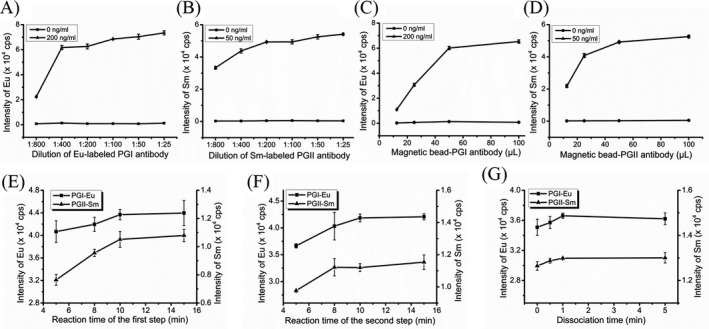
Optimization of the established immunoassay for pepsinogen I (PGI) and pepsinogen II. A, Optimization of the labeling antibody concentration for PGI; B, optimization of the labeling antibody concentration for PGII; C, optimization of the capture antibody concentration for PGI; D, optimization of the capture antibody concentration for PGII; E, optimization of reaction time for the first incubation; F, optimization of reaction time for the second incubation; and G, optimization of dissociation time
Furthermore, the overall time of the assays for PGI and PGII analysis was evaluated by PGI standards (200 ng/mL) and PGII standards (50 ng/mL). For the sandwich immunoassay format, the reaction time of 5, 8, 10, and 15 minutes for the first and second incubation (as shown in Figure 1) was investigated, respectively. The time with the highest signal that appeared first was selected. Thus, the reaction time of the first incubation was 10 minutes for PGI and PGII (Figure 2E), and the reaction time of the second incubation was also 10 minutes for PGI and PGII (Figure 2F). Besides, the dissociation time that the Eu and Sm were released to the supernatant was 1 minute (Figure 2G).
3.2. Standard curves of the established assays
The standard curves of PGI and PGII were shown in Figure 3. The regression equations for the calibration curves were as follows: y = (427.14 ± 12.78) x – (142.62 ± 60.47) for PGI (R 2 = .9938) and y = (1030.13 ± 36.90) x – (195.12 ± 142.85) for PGII (R 2 = .9911), respectively. A good linear relationship was obtained in the concentration range of 3‐200 ng/mL for PGI and 1‐60 ng/mL for PGII, respectively. The LOD of the assay, defined as three times the standard deviation of the blank, was 1.8 ng/mL for PGI and 0.3 ng/mL for PGII, respectively.
Figure 3.
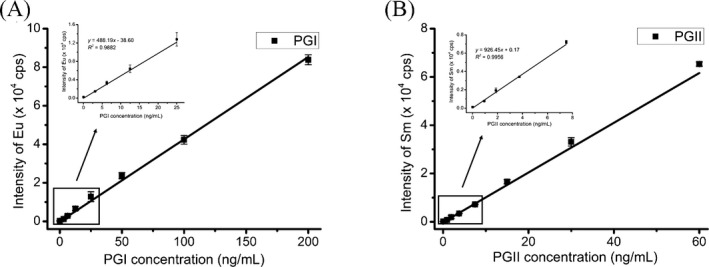
The standard curves of pepsinogen I
3.3. Recovery
Four clinical serum samples with known concentration of PGI and PGII were used in this recovery assays. The original concentration of PGI and PGII was 43.98, 34.37, 32.10, 32.54 ng/mL and 8.28, 4.31, 6.77, 6.50 ng/mL, respectively. The concentrations of spiked PGI and PGII standards were 11.30, 47.40 ng/mL and 4.70, 14.70 ng/mL, respectively. As shown in Table 1, the average recoveries were 101.41 ± 6.74% (in the range of 92.95%‐112.02%) for PGI and 101.47% ± 4.20% (in the range of 95.74%‐108.16%) for PGII, respectively. (The average recoveries obtained by the proposed method using four concentrations of serum samples were 97.97% ± 3.41%, 104.68% ± 10.38%, 101.80% ± 4.18%, 101.19% ± 11.67% for PGI and 103.8% ± 0.61%, 104.08% ± 5.77%, 96.85% ± 1.57%, 101.11% ± 4.56% for PGII, respectively). The results show that the proposed assay was low matrix effect in the serum.
Table 1.
Recoveries of pepsinogen I (PGI) and pepsinogen II (PGII) determined by the proposed method (n = 2)
| Sample | PGI | PGII | ||||||
|---|---|---|---|---|---|---|---|---|
|
Added (ng/mL) |
Determined Mean ± SD (ng/mL) |
Average recovery (%) |
CV (%) |
Added (ng/mL) |
Determined Mean ± SD (ng/mL) |
Average recovery (%) |
CV (%) |
|
| 1 | 11.30 | 11.38 ± 1.05 | 100.38 | 9.23 | 4.70 | 4.90 ± 0.20 | 104.26 | 4.08 |
| 47.40 | 45.34 ± 1.86 | 95.56 | 4.10 | 14.70 | 15.20 ± 0.32 | 103.40 | 2.11 | |
| 2 | 11.30 | 11.03 ± 0.64 | 97.34 | 5.66 | 4.70 | 4.70 ± 0.31 | 100.00 | 6.60 |
| 47.40 | 53.15 ± 2.53 | 112.02 | 4.76 | 14.70 | 15.90 ± 0.58 | 108.16 | 3.65 | |
| 3 | 11.30 | 11.87 ± 0.70 | 104.75 | 5.90 | 4.70 | 4.50 ± 0.20 | 95.74 | 4.44 |
| 47.40 | 46.90 ± 1.83 | 98.84 | 3.90 | 14.70 | 14.40 ± 0.54 | 97.96 | 3.75 | |
| 4 | 11.30 | 12.40 ± 1.10 | 109.42 | 8.87 | 4.70 | 4.90 ± 0.11 | 104.26 | 2.24 |
| 47.40 | 44.10 ± 2.16 | 92.95 | 4.90 | 14.70 | 14.40 ± 0.21 | 97.96 | 1.46 | |
3.4. Precision
The precision was evaluated by measuring two clinical serum samples with low and high concentration of PGI and PGII. As shown in Table 2, the intra‐assay coefficients of variation (CV) for PGI were 1.79% and 3.59%, and the inter‐assay CV were 9.03% and 5.38%. The intra‐assay CV for PGII were 3.78% and 2.44%, and the inter‐assay CV were 5.57% and 3.81%. All of the intra‐assay CV were <10%, and inter‐assay CV were <15%, indicating that the established method has good precision.
Table 2.
Precision of pepsinogen I (PGI) and pepsinogen II (PGII) determined by the proposed method
| PGI | PGII | ||||||
|---|---|---|---|---|---|---|---|
|
Average (ng/mL) |
SD (ng/mL) |
CV (%) |
Average (ng/mL) |
SD (ng/mL) |
CV (%) |
||
|
Intra‐assay (n = 8) |
Low | 37.20 | 1.79 | 4.83 | 6.35 | 0.24 | 3.78 |
| High | 77.28 | 3.59 | 4.64 | 12.74 | 0.31 | 2.44 | |
|
Inter‐assay (n = 20) |
Low | 38.28 | 3.46 | 9.03 | 6.32 | 0.35 | 5.57 |
| High | 77.11 | 4.15 | 5.38 | 12.78 | 0.49 | 3.81 | |
3.5. Interference and specificity
Two clinical serum samples with known concentration of PGI and PGII were used in this interference test. The original concentration of PGI and PGII were 106.18, 47.21 ng/mL and 7.34, 51.67 ng/mL, respectively. The concentrations of spiked triglyceride, bilirubin and hemoglobin were 2.5, 200, and 500 μg/mL, respectively. As shown in Table 3, the biases were in the range of 0.10%‐8.04% for PGI and 0.09%‐3.30% for PGII, respectively. All of the biases were <10%, showing that the interferants had little effect on serum detection.
Table 3.
Interference test of pepsinogen I (PGI) and pepsinogen II (PGII) determined by the proposed method (n = 2)
| Interference | Sample | PGI | PGII | ||||
|---|---|---|---|---|---|---|---|
|
Determined (ng/mL) |
Difference (ng/mL) |
Biases (%) |
Determined (ng/mL) |
Difference (ng/mL) |
Biases (%) |
||
| Triglyceride | 1 | 109.01 | 2.83 | 2.67 | 7.18 | ‐0.16 | 2.14 |
| (2.5 mg/mL) | 2 | 50.99 | 3.78 | 8.02 | 53.37 | 1.70 | 3.30 |
| Bilirubin | 1 | 106.07 | −0.11 | 0.10 | 7.55 | 0.21 | 2.89 |
| (200 μg/mL) | 2 | 51.00 | 3.80 | 8.04 | 52.67 | 1.01 | 1.95 |
| Hemoglobin | 1 | 102.83 | −3.34 | 3.15 | 7.11 | −0.24 | 3.20 |
| (500 μg/mL) | 2 | 48.39 | 1.18 | 2.51 | 51.62 | −0.04 | 0.09 |
Furthermore, the specificity of PGI and PGII was also evaluated by investigation of antibody cross‐reactivity. For the PGI, the high concentration of PGII standard (50 ng/mL) was measured by PGI, and the value of determined PGI was <1.0 ng/mL. For the PGII, the high concentration of PGI standard (200 ng/mL) was measured by PGII, and the value of determined PGII was <0.5 ng/mL. The determined concentrations of PGI and PGII were <2.0 ng/mL, indicating the high specificity.
3.6. Comparison with commercial assays
To further validate the proposed method for clinical application, 103 clinical serum samples were analyzed by the established method and commercial CLIA kit. As shown in Figure 4, good correlations were obtained between the proposed method and CLIA. The regression equations: y = (0.9085 ± 0.0268) x + (6.0535 ± 1.9399) for PGI (r = .9588) and y = (0.9582 ± 0.0165) x + (0.4982 ± 0.2208) for PGII (r = .9853), respectively. Furthermore, the results obtained by two methods were also statistically analyzed by the paired t test. As a result, the P values were .6816 for PGI and .6607 for PGII, respectively. The P value was <.05, showing that there is no significant difference between the two methods. The results indicated that the proposed method could serve for simultaneous determination of PGI and PGII in clinical laboratory.
Figure 4.
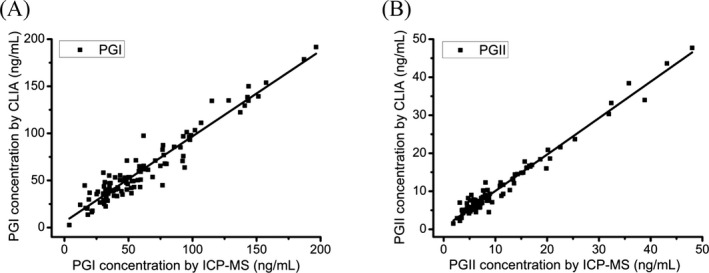
Comparisons of the proposed method and CLIA method for determination of PGI (A) and PGII (B)
4. DISCUSSION
Over the past two decades, the element tagged immunoassay combined with ICP‐MS detection has been fully demonstrated the multiplex analysis in analytical methodology. Compared with the commonly used immunoassay, this method provides several benefits, such as multiple labeling, high sensitivity, wide linear range, and no background interference. However, this method has not been used in routine clinical analysis, and only a few studies have reported the clinical application of this method. Thus, this method was performed by simultaneous determination of PGI and PGII in the human serum samples.
In this study, a dual‐label ICP‐MS‐linked immunoassay, using stable isotope element of Sm and Eu, was developed for the simultaneous determination of PGI and PGII. The LOD and linear range of the assay were 1.8 ng/mL, 3‐200 ng/mL for PGI, and 0.3 ng/mL, 1‐60 ng/mL for PGII, respectively. The sensitivity and linear range were similar to that of the traditional immunoassay, such as ELISA,9 CLIA,11 ECLIA,12, 22 TRFIA,23 and FIA.24 The performance of the established immunoassay could satisfy the analysis of almost all of the clinical serum samples. The average recoveries (101.41% ± 6.74% for PGI and 101.47% ± 4.20% for PGII), and intra‐ and inter‐assay CVs (<10%) fully met the requirements of clinical laboratory. The interference and cross‐reactivity test indicated that there was no obvious cross‐reaction with the common interfering substances. When compared with the commercial CLIA method, 103 clinical serum samples were measured by both methods. High correlation coefficients were obtained between the proposed method and CLIA method, which indicated that the proposed assay could be used for simultaneous determination of PGI and PGII in a single analysis.
Furthermore, the overall time for sample analysis was also investigated, which was an important factor in determining whether the new method was fit for clinical analysis. In this study, the two‐step dual‐label assay (as shown in Figure 1) was established. As we all know, the reaction between antigen and antibody was very quick. The incubated time was 10 minutes for the first reaction of magnetic beads‐antibody and antigen, and the second reaction of antigen and Eu/Sm‐labeled antibody, respectively (Figure 2E,F). Besides, the dissociation time of 0, 0.5, 1, and 5 minutes was investigated for Eu and Sm released to the supernatant. The antibody‐antigen complexes were easily destroyed with the pH < 2.0. The dissociation time that the Eu/Sm were released to the supernatant of HNO3 solution was less than 1 minute (Figure 2G). The detection time for simultaneous readout of Eu and Sm signal was less than 1 minute. As for the washing steps, the washing time of five times was about 3 minutes. As a result, the total time taken for a single analysis was about 25 minutes, which was faster than the manual immunoassay, such as ELISA. As for the automated CLIA and ECLIA kit, the total time of the assay was about 20 minutes. With the development of automation, we believe that the total time cost with ICP‐MS based immunoassay will eventually be similar to CLIA and ECLIA.
In conclusion, a rapid, sensitive, and dual‐label immunoassay combined with ICP‐MS detection was developed for the simultaneous determination of PGI and PGII, which was suitable and promising for the diagnosis of gastric cancer. This study could expand the application of mass spectrometry in the clinical laboratories. Furthermore, the experimental procedure is almost identical to the traditional immune step and easily accepted by the analyst of the clinic laboratory. This method is expected to be used as a routine clinical analysis tool, which will provide a new method for multiplex bioassays and clinical diagnosis for individual therapy.
Jiang W, Sun G, Zhang Y, et al. Simultaneous determination of gastric cancer biomarkers pepsinogen PGI/PGII using element tagged immunoassay coupled with inductively coupled plasma mass spectrometry detection. J Clin Lab Anal. 2020;34:e23287 10.1002/jcla.23287
Wencan Jiang and Gongwei Sun are authors have contributed equally for this paper.
Contributor Information
Biao Huang, Email: wangcbin301@163.com, Email: jswxhb@163.com.
Chengbin Wang, Email: wangcbin301@163.com.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27:v38‐v49. [DOI] [PubMed] [Google Scholar]
- 3. Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9(4):245‐253. [DOI] [PubMed] [Google Scholar]
- 4. Tu H, Sun L, Dong X, et al. A serological biopsy using five stomach‐specific circulating biomarkers for gastric cancer risk assessment: a multi‐phase study. Am J Gastroenterol. 2017;112(5):704‐715. [DOI] [PubMed] [Google Scholar]
- 5. Tong Y, Wu Y, Song Z, Yu Y, Yu X. The potential value of serum pepsinogen for the diagnosis of atrophic gastritis among the health check‐up populations in China: a diagnostic clinical research. BMC Gastroenterol. 2017;17:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang X‐M, Li J‐X, Zhang G‐Y, Li X‐H, Gu H. The value of serum pepsinogen levels for the diagnosis of gastric diseases in Chinese Han people in midsouth China BMC Gastroenterol. 2014;14(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang Y‐K, Yu J‐C, Kang W‐M, et al. Significance of serum pepsinogens as a biomarker for gastric cancer and atrophic gastritis screening: a systematic review and meta‐analysis. PLoS ONE. 2015;10(11):e0142080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Agreus L, Kuipers EJ, Kupcinskas L, et al. Rationale in diagnosis and screening of atrophic gastritis with stomach‐specific plasma biomarkers. Scand J Gastroenterol. 2012;47(2):136‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Konishi N, Matsumoto K, Hiasa Y, Kitahori Y, Hayashi I, Matsuda H. Tissue and serum pepsinogen‐I and pepsinogen‐II in gastric‐cancer identified using immunohistochemistry and rapid ELISA. J Clin Pathol. 1995;48(4):364‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang B, Xiao HL, Zhang XR, Zhu L, Liu HY, Jin J. Ultrasensitive detection of pepsinogen I and pepsinogen II by a time‐resolved fluoroimmunoassay and its preliminary clinical applications. Analytica Chimica Acta. 2006;571(1):74‐78. [DOI] [PubMed] [Google Scholar]
- 11. Cho E‐J, Kim H‐K, Jeong T‐D, et al. Method evaluation of pepsinogen I/II assay based on chemiluminescent immunoassays and comparison with other test methods. Clinica Chimica Acta. 2016;452:149‐154. [DOI] [PubMed] [Google Scholar]
- 12. Ogasawara D, Hirano Y, Yasukawa T, et al. Electrochemical microdevice with separable electrode and antibody chips for simultaneous detection of pepsinogens 1 and 2. Biosens Bioelectron. 2006;21(9):1784‐1790. [DOI] [PubMed] [Google Scholar]
- 13. Liu R, Zhang S, Wei C, Xing Z, Zhang S, Zhang X. Metal stable isotope tagging: renaissance of radioimmunoassay for multiplex and absolute quantification of biomolecules. Acc Chem Res. 2016;49(5):775‐783. [DOI] [PubMed] [Google Scholar]
- 14. Liu R, Wu P, Yang L, Hou X, Lv Y. Inductively coupled plasma mass spectrometry‐based immunoassay: a review. Mass Spectrom Rev. 2014;33(5):373‐393. [DOI] [PubMed] [Google Scholar]
- 15. Giesen C, Waentig L, Panne U, Jakubowski N. History of inductively coupled plasma mass spectrometry‐based immunoassays. Spectrochim Acta B. 2012;76:27‐39. [Google Scholar]
- 16. Zhang C, Wu FB, Zhang YY, Wang X, Zhang XR. A novel combination of immunoreaction and ICP‐MS as a hyphenated technique for the determination of thyroid‐stimulating hormone (TSH) in human serum. J Anal Atom Spectrom. 2001;16(12):1393‐1396. [Google Scholar]
- 17. Zhang SC, Zhang C, Xing Z, Zhang XR. Simultaneous determination of alpha‐fetoprotein and free beta‐human chorionic gonadotropin by element‐tagged immunoassay with detection by inductively coupled plasma mass Spectrometry. Clin Chem. 2004;50(7):1214‐1221. [DOI] [PubMed] [Google Scholar]
- 18. Bendall SC, Simonds EF, Qiu P, et al. Single‐cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332(6030):687‐696.21551058 [Google Scholar]
- 19. Sun G, Huang B, Zhang Y, et al. A combinatorial immunoassay for multiple biomarkers via a stable isotope tagging strategy. Chem Commun. 2017;53(97):13075‐13078. [DOI] [PubMed] [Google Scholar]
- 20. Han G, Spitzer MH, Bendall SC, Fantl WJ, Nolan GP. Metal‐isotope‐tagged monoclonal antibodies for high‐dimensional mass cytometry. Nat Protoc. 2018;13(10):2121‐2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou B, Zhang J, Lv Z, Fan J, Zhang Y, Huang B. Simultaneous determination of free and total prostate‐specific antigen by a magnetic particle‐based time‐resolved fluoroimmunoassay. J Clin Lab Anal. 2017;31(6):e22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xie Y, Zhi X, Su H, et al. A novel electrochemical microfluidic chip combined with multiple biomarkers for early diagnosis of gastric cancer. Nanoscale Res Lett. 2015;10:477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fan J, Xiao H, Zhang J, et al. A magnetic nanoparticle‐labeled immunoassay with europium and samarium for simultaneous quantification of serum pepsinogen I and II. Br J Biomed Sci. 2017;74(3):127‐132. [DOI] [PubMed] [Google Scholar]
- 24. Li K, Li X, Fan Y, Yang C, Lv X. Simultaneous detection of gastric cancer screening biomarkers plasma pepsinogen I/II using fluorescent immunochromatographic strip coupled with a miniature analytical device. Sensor Actuat B Chem. 2019;286:272‐281. [Google Scholar]


