Fig. 3. Monobodies that modulate biological functions of their respective targets and their crystal structures.
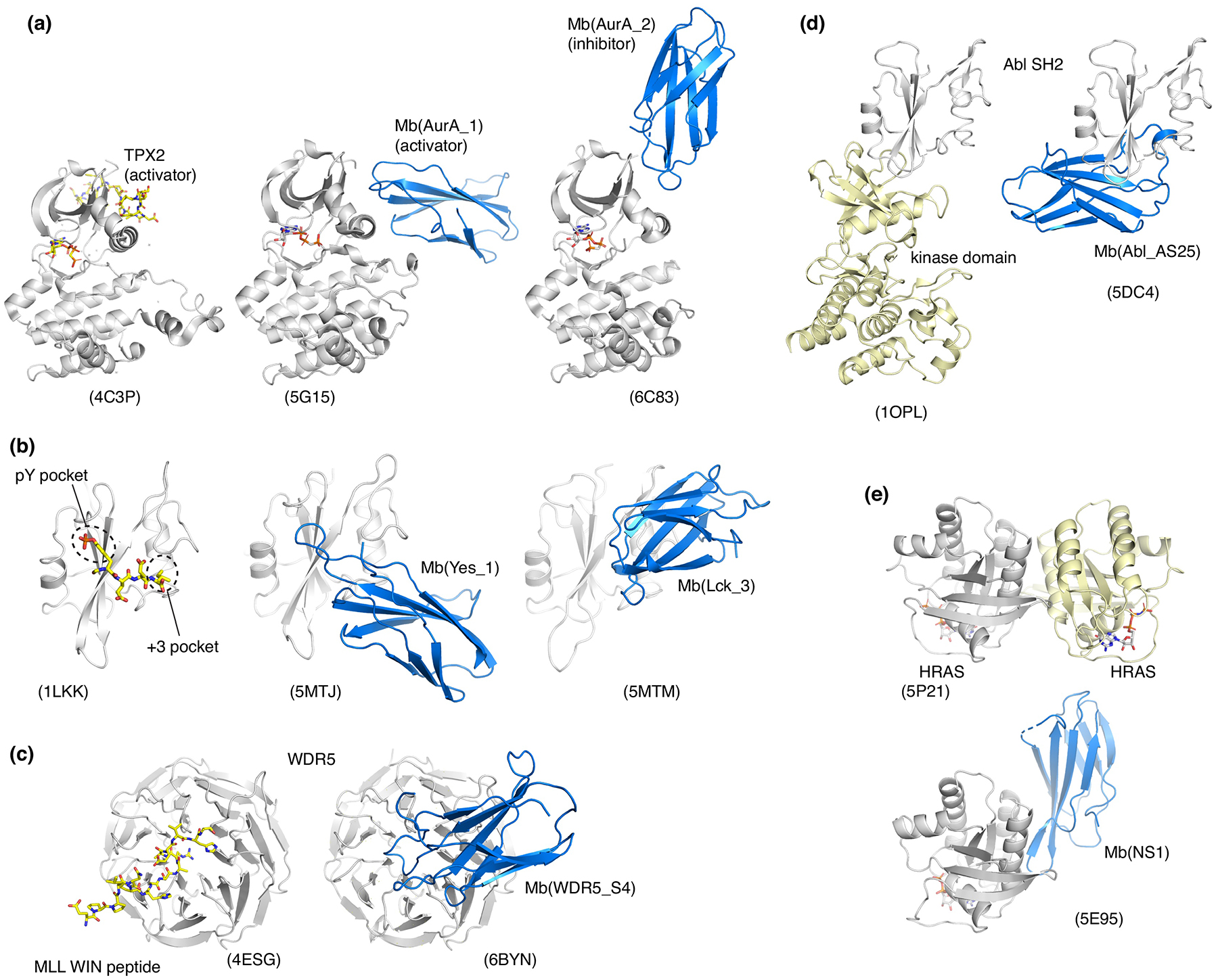
(a) Allosteric activator and inhibitor of Aurora A kinase. (b) Competitive inhibitors of the interaction between SH2 domains and pY peptides. (c) Competitive inhibitor of the interaction between WDR5 and a fragment of MLL1. (d) Allosteric inhibitor targeting the interface between the Bcr-Abl SH2 and kinase domains. (e) Allosteric inhibitor targeting the self-association surface of HRAS.
