Figure 1.
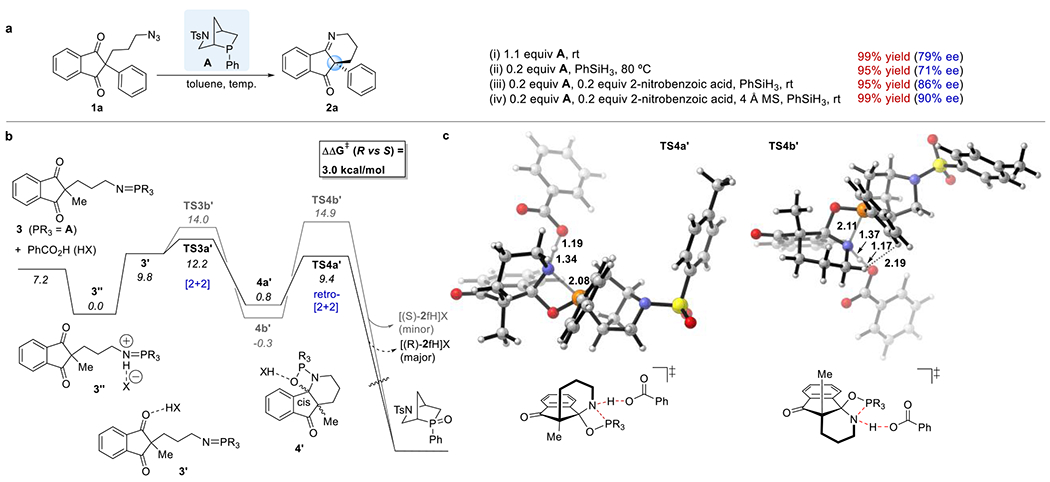
Rationalization of the observed enantioselectivity. (a) Optimization of the reaction conditions. (b) Free energy profile for the formation of the major (R, black lines) and minor (S, gray lines) enantiomers of the iminoketone in the aza-Wittig reaction of the iminophosphorane 3 in the presence of benzoic acid. (c) Structures of the transition states TS4a′ and TS4b′, highlighting the key stereodetermining interactions. Calculations performed with M06-2X/6-311++G(d,p)-SMD(toluene)//B3LYP/6-31G(d)-SMD(toluene). Numbers in italics are Gibbs free energies in kcal/mol.
