Figure 1. Multiple molecular level activities cooperate to organize and shape mitotic chromosomes.
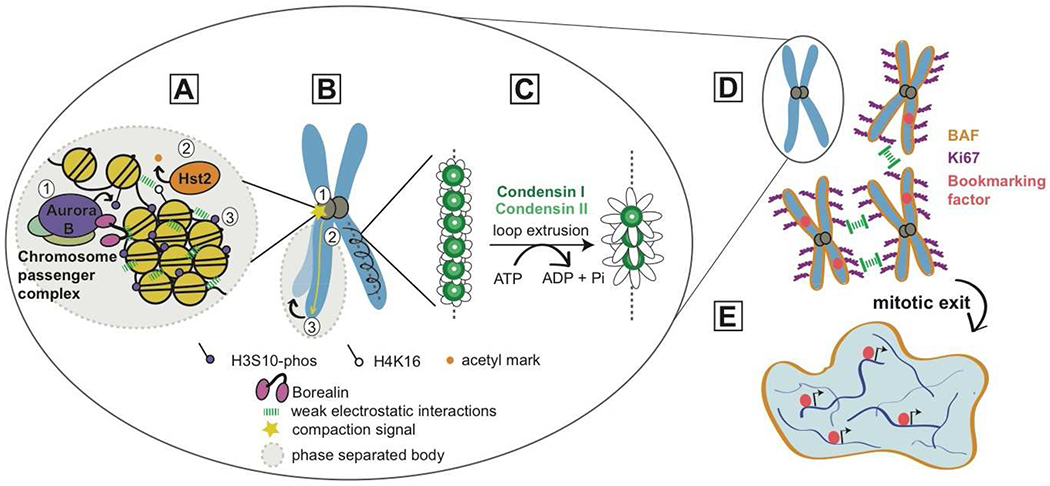
(A) Chromatin compaction at mitotic onset is driven by a cascade of histone post translational modifcations (PTMs) initiated by Aurora B, the kinase subunit of the chromosome passenger complex (CPC). Aurora B phosphorylates S10 of histone H3 (1), which recruits the deacetylase Hst2 that acts on histone H4 K16 (2). These changes result in increased multivalent interactions among neighboring nucleosomes, driven in part by an unstructured domain of Borealin, another subunit of the CPC (3). Ultimately, these interactions result in increased compaction of chromatin, which could be driven by phase separation.
(B) In the model proposed in Kruitwagen et al. [14], the compaction signal mediated by Aurora B initiates at the centromere (1) and spreads along the chromosome arm (2), resulting in a shortened, more compact chromosome (3).
(C) In the model proposed in Gibcus et al. [1], condensin I and II form loops of DNA perpendicular to the central axis. In this model, loop extrusion by the condensins creates larger loops of DNA, and thus axial shortening of the chromosome arm.
D-E) Interchromosomal organization is driven by two DNA binding molecules: Ki67 and BAF. Ki67 helps individualize chromosomes by acting as a “surfactant brush”, repelling chromosomes from one another. Upon mitotic exit, formation of a complete nucleus is aided by BAF, which crosslinks chromosomes at their periphery, preventing chromosome loss. Finally, mitotic bookmarking factors that remain on mitotic chromosomes help re-initiate transcriptional programs upon re-entry into interphase.
