Figure 3. The core components controlling ferroptosis.
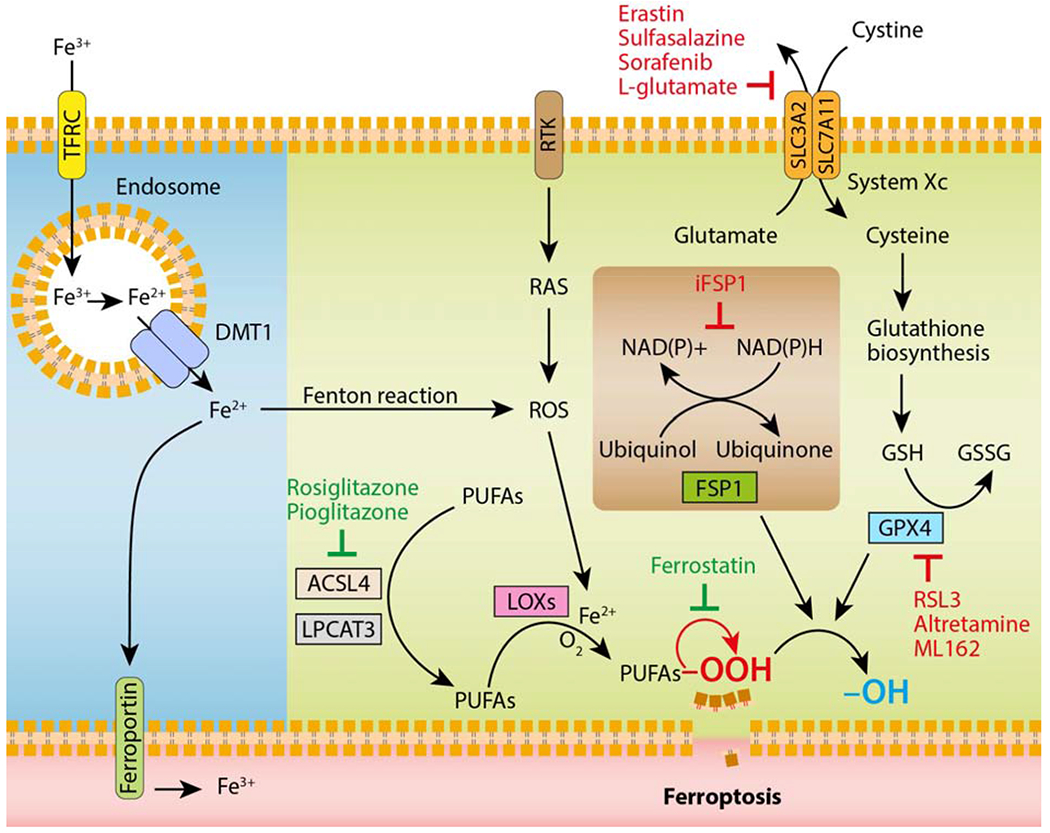
Glutathione (GSH) is a tripeptide essential for preventing damage caused by ROS, such as lipid peroxides. It is synthesized continuously from cysteine, glutamate and glycine. Cellular availability of cysteine is the limiting step for GSH synthesis. The Xc− transporter, which consists of two subunits belonging to the family of solute transporters (SLC3A2 and SLC7A11), is responsible for the uptake of extracellular cystine, the precursor of cysteine. GSH provides electrons to the key regulator of ferroptosis, GPX4, which reduces lipid peroxides (-OOH) in plasma membranes to the corresponding alcohols (-OH). Recently, the enzymes ACSL4 and LPCAT3 that are directly involved in shaping the cellular lipid composition, have been shown to sensitize cells to ferroptosis. Oxidation of lipid bilayers during ferroptosis occurs both in an enzymatic (i.e., LOXs, lipoxygenases) and non-enzymatic (i.e., radical-mediated autoxidative; symbolized by the red arrow) manner. Alternatively, FSP1 can suppress ferroptosis by ubiquinone, the reduced form of ubiquinol, which traps lipid peroxyl radicals. FSP1 catalyses the regeneration of ubiquinone using NAD(P)H. Cellular iron homeostasis, which is dependent on the coordination of iron uptake (i.e., TFRC) and export (i.e., ferroportin), directly regulates ferroptosis through Fenton reactions and generation of ROS. Fe3+ is reduced to Fe2+ by metalloreductases in the endosome, and the divalent metal transporter 1 (DMT1) mediates the transport of Fe2+ from the endosome into a labile iron pool in the cytoplasm. Receptor tyrosine kinase (RTK) activates the oncogene RAS, which is known to induce oxidative stress through generation of ROS. Ferroptosis-inducing drugs are depicted in red, whereas ferroptosis inhibitors are shown in green.
