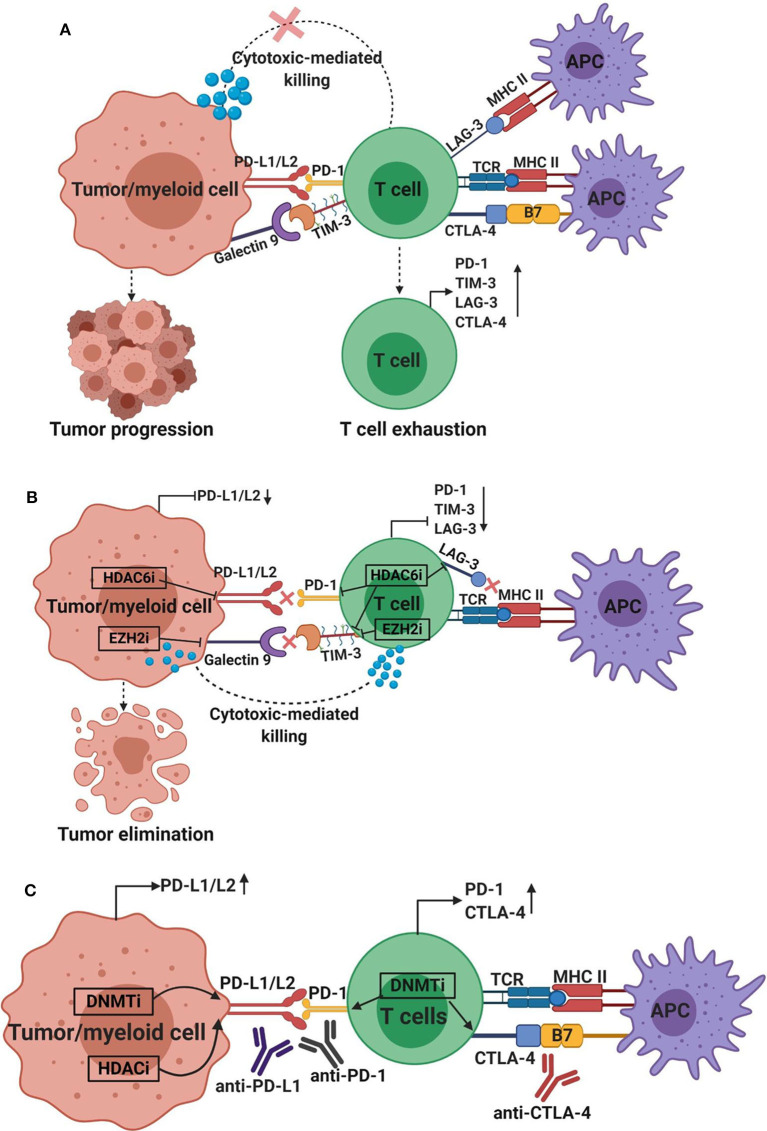
Figure 5.
Effect of epigenetic modifiers on the expression of immune checkpoints and their ligands in the tumor microenvironment. The interaction between co-inhibitory immune checkpoints on immune cells and their ligands on tumor cells or myeloid cells results in tumor progression, immunosuppression and T cell exhaustion characterized by increased expression of immune checkpoints, including PD-1, CTLA-4, TIM-3, and LAG-3, and loss of effector functions, such as cytokine release and cell-mediated cytotoxicity. The interactions between PD-1, TIM-3, CTLA-4, and LAG-3 on T cells with their respective ligands PD-L1/PD-L2, galectin-9, B7 ligands or MHC II on tumor cells/myeloid cells or APC, generate signals that inhibit T cell activation/proliferation (A). Depending on the tumor microenvironment and tumor type, the application of epigenetic modifiers can downregulate or upregulate the expression of immune checkpoints and their ligands. The application of HDAC6 inhibitor (HDAC6i) can downregulate the expression of PD-L1/2, PD-1, TIM-3, and LAG-3, and EZH2 inhibitor (EZH2i) can downregulate the expression of galectin-9 and TIM-3 (B), indicating the potential benefits of using these modifiers to enhance anti-tumor immune responses and promote tumor cell killing. On the other hand, application of DNMT inhibitor (DNMTi; azacytidine or decitabine) can upregulate the expression of PD-L1/2, PD-1 and CTLA-4, and HDAC inhibitor (HDACi; vorinostat; or panobinostat) can upregulate the expression of PD-L1/2 (C), suggesting the potential benefit of combining epigenetic modifies with immune checkpoint inhibitors, such as anti-CTLA-4, anti-PD-1 or anti-PD-L1, to increase the sensitivity of the host immune response and promote more potent anti-tumor immunity.