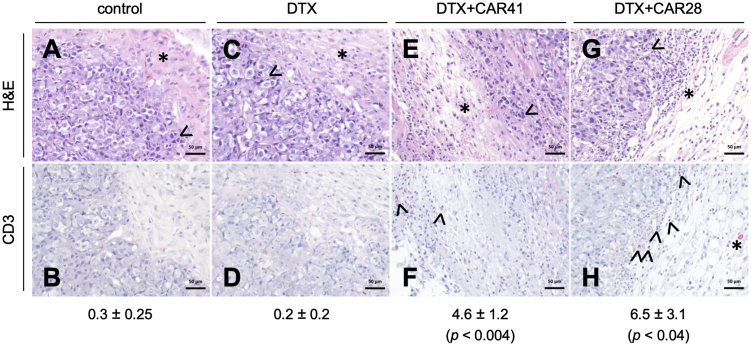
Figure 5.
Histology
At the end of the combined docetaxel (DTX) and CAR T cell therapy, tumor slices of indicated groups were subjected to histopathological analysis by hematoxylin and eosin (H&E) staining (A, C, E, and G) or immunohistochemical analysis using anti-human CD3 that highlights human CD3+ T cells in red (B, D, F, and H). (A and B) Untreated group (control) with few nuclear condensations (arrowhead, exemplary) of tumor cells near the tumor margin, low cellular density in the tumor microenvironment (TME), consisting of connective tissue with few macrophages (asterisk), and absence of CD3+ T cell infiltrates. (C and D) DTX-treated group with nuclear condensations and cellular vacuolization but higher cellularity in the TME due to infiltration of monocytes and neutrophil granulocytes (asterisk), accompanied by stromal edema, and absence of CD3+ T cells. (E and F) Combination therapy group (DTX and CAR41 T cells) with presence of TME with high stromal edema and stroma cellularity due to increased monocytes, macrophages, and lymphocytes infiltrating the connective tissue (asterisk in E), accompanied by tumor necrosis at the tumor-stroma border (arrowhead in E), and few infiltrating CD3+ T cells in the TME (arrowheads in F). (G and H) Combination therapy group (DTX and CAR28 T cells) with presence of TME with high stromal edema and stroma cellularity (asterisk in G), accompanied by tumor necrosis zone (arrowhead in G), and high amount of infiltrating CD3+ T cells (arrowheads in H) and macrophage with CD3+ cytoplasm (asterisk in H). The average numbers ± standard error of the mean of infiltrating CD3 cells per tumor slice is indicated below. p values are indicated for comparison between the DTX group versus the DTX+CAR41 or DTX+CAR28 groups.