Figure 1. Engineering hybrid Patz operator-promoter variants for a cyanuric acid-inducible whole-cell E. coli sensor.
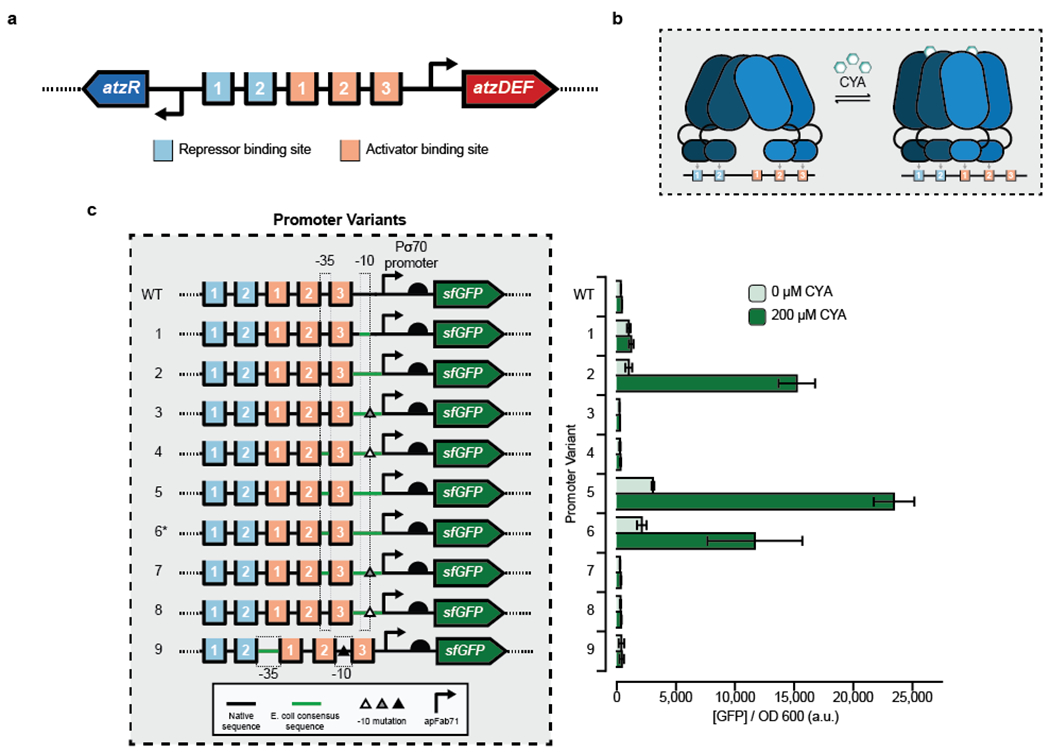
(a) The divergent PatzDEF-PatzR operon regulated by AtzR includes 2 repressor binding sites (light blue) that negatively autoregulate atzR expression and 3 activator binding sites (orange) that regulate atzDEF transcription. (b) AtzR, a LysR-Type Transcriptional Regulator (LTTR), is composed of a dimer-of-dimers. In the absence of cyanuric acid (CYA), AtzR binds to repressor binding sites 1 and 2, and activator binding sites 2 and 3. In the presence of CYA, a dimer of AtzR preferentially occupies activator binding sites 1 and 2, thereby allowing transcriptional machinery to access the promoter. This “sliding dimer” mechanism is typical of LTTRs. (c) Hybrid promoter designs to create a whole-cell E. coli sensor for CYA by combining native sequence from Pseudomonas sp. strain ADP1 at the 5’ end, a strong E. coli promoter (apFab71) at the 3’ end and mutations to the transcriptionally-sensitive 3’ end of the −10 sequence (triangles). Transcriptional activation in the absence and presence of 200uM CYA was monitored by production of a downstream superfolder green fluorescent protein (sfGFP). A high-copy plasmid (a variant of the SC101origin of replication) was used for all promoter variants except promoter 6* which used a low-copy plasmid (p15a origin of replication). Bars indicate the average of experimental triplicate with error bars depicting 1 standard deviation. Measurements were taken after 16 hours growth at 37°C.
