Figure 4. Proof-of-concept for field-deployable cyanuric acid sensing using a cell-free biosensor.
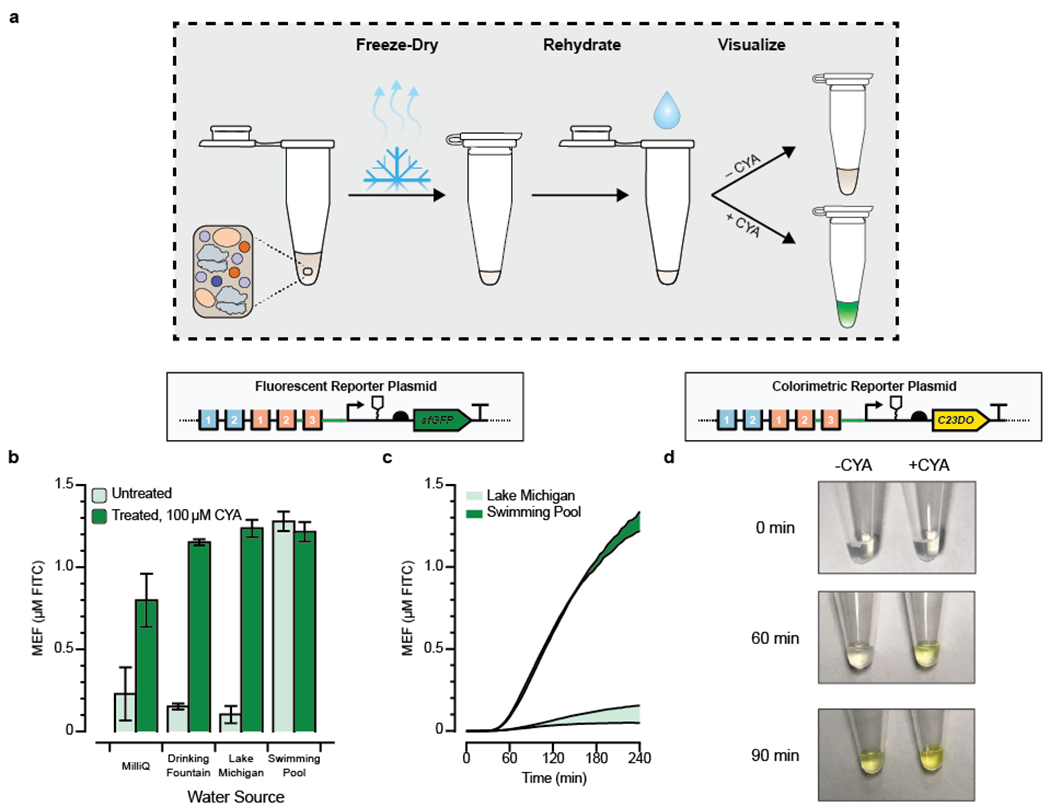
(a) Optimized cell-free reactions are prepared without cyanuric acid (CYA), freeze-dried, and then rehydrated with unfiltered environmental water samples. (b) CYA in environmental water samples can be detected from freeze-dried cell-free reactions. Pre-mixed cell-free reactions were freeze-dried and rehydrated with 90% of an environmental water sample and 10% of either MQ water (OFF) or 10% MQ water spiked with a final concentration of 100 μM CYA (ON). sfGFP fluorescence is specifically induced by CYA in every sample except the pool water, which is expected to already contain CYA even in the OFF state. The kinetics for all conditions is presented in Supp. Fig. 5. (c) Time course comparisons of the OFF state (No CYA added) from panel B, for the unfiltered environmental water samples that were not spiked with CYA. Pool water can be distinguished against lake water in less than one hour. (d) Time course comparison for activation of cell-free reaction driving expression of catechol 2,3-dioxygenase (C23DO), an enzymatic reporter that cleaves its colorless substrate catechol into a yellow product, cis,cis-muconic acid. CYA was added at a concentration of 600 μM in the ON state to a fresh cell-free reaction incubated at 30°C and monitored for 90 minutes. Pictures are cropped representative images from the experiment, which was done in triplicate. Uncropped images are in Supp. Fig. 7. Error bars in b and c represent one standard deviation from three technical replicates. Fluorescence in b and c is reported standardized to mean equivalent fluorescence (MEF) of fluorescein isothiocyanate based on a previously developed standard.
