Figure 5. Glycosylation at N62 of GRP94 Is a Key Regulator in the Formation of the HMW GRP94 Variant and Is Important for Its Oncogenic Activity.
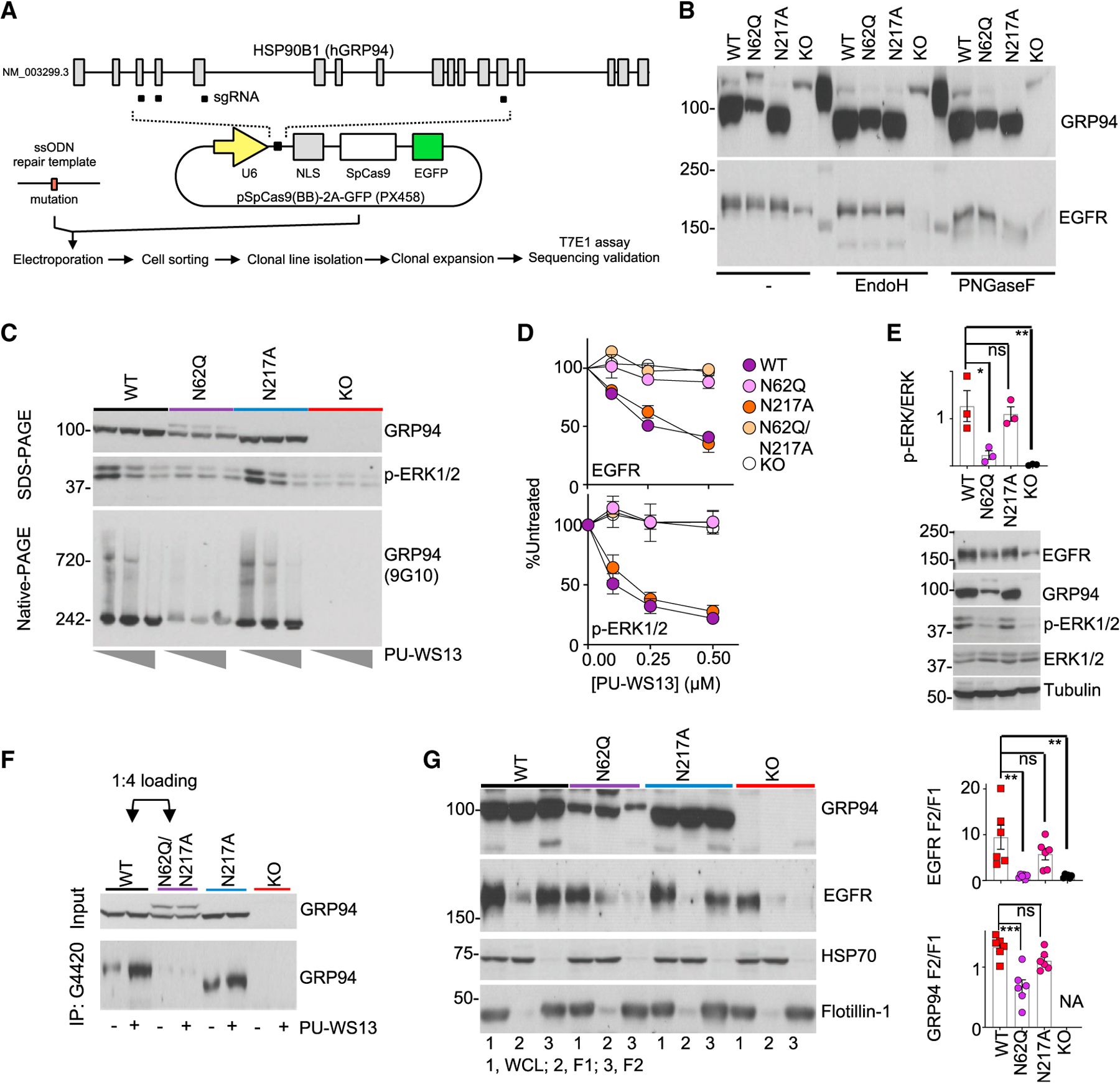
(A) Schematic for clone generation and validation. MDA-MB-468 cells (WT) and clones containing the indicated GRP94 mutants were then used for the analyses below.
(B) The effect of GRP94 N-glycan mutagenesis or KO evaluated on glycosylation load.
(C) GRP94 HMW formation (measured by blotting for GRP94 HMW species on native-PAGE and ERK activity on SDS-PAGE) and its sensitivity to PU-WS13, in cells treated for 24 h with 0, 0.25, or 1 μM PU-WS13.
(D) Steady-state levels of EGFR and activity of its downstream signaling (measured by p-ERK levels) in cells treated for 24 h with PU-WS13. Graph, mean ± SEM; quantification of 3 western blot analyses.
(E) Baseline activity of EGFR-downstream signaling measured by western blot analysis of p-ERK and ERK. Graph, mean (n = 3). Error bar, SEM; one-way ANOVA with Dunnett’s post hoc, *p < 0.05, **p < 0.01.
(F) GRP94 immunocapture in clones pre-treated for 4 h with vehicle (−) or PU-WS13 (2.5 μM). Four times more lysate from the N62Q-containing clone was loaded to normalize for the GRP94 input.
(G) Cellular localization of EGFR and GRP94. 1, WCL; 2, F1; 3, F2 fractions. Tubulin, HSP70, and Flotillin-1 are controls for equal loading and for cellular fractionation purity; NA, not available. Graph, mean ± SEM; one-way ANOVA with Dunnett’s post hoc; n = 6 individual experiments. ***p < 0.001, **p < 0.01.
See also Figure S5.
