Figure 4. Copper-Mediated Electrochemical Dehydrogenative Homocoupling of 1.
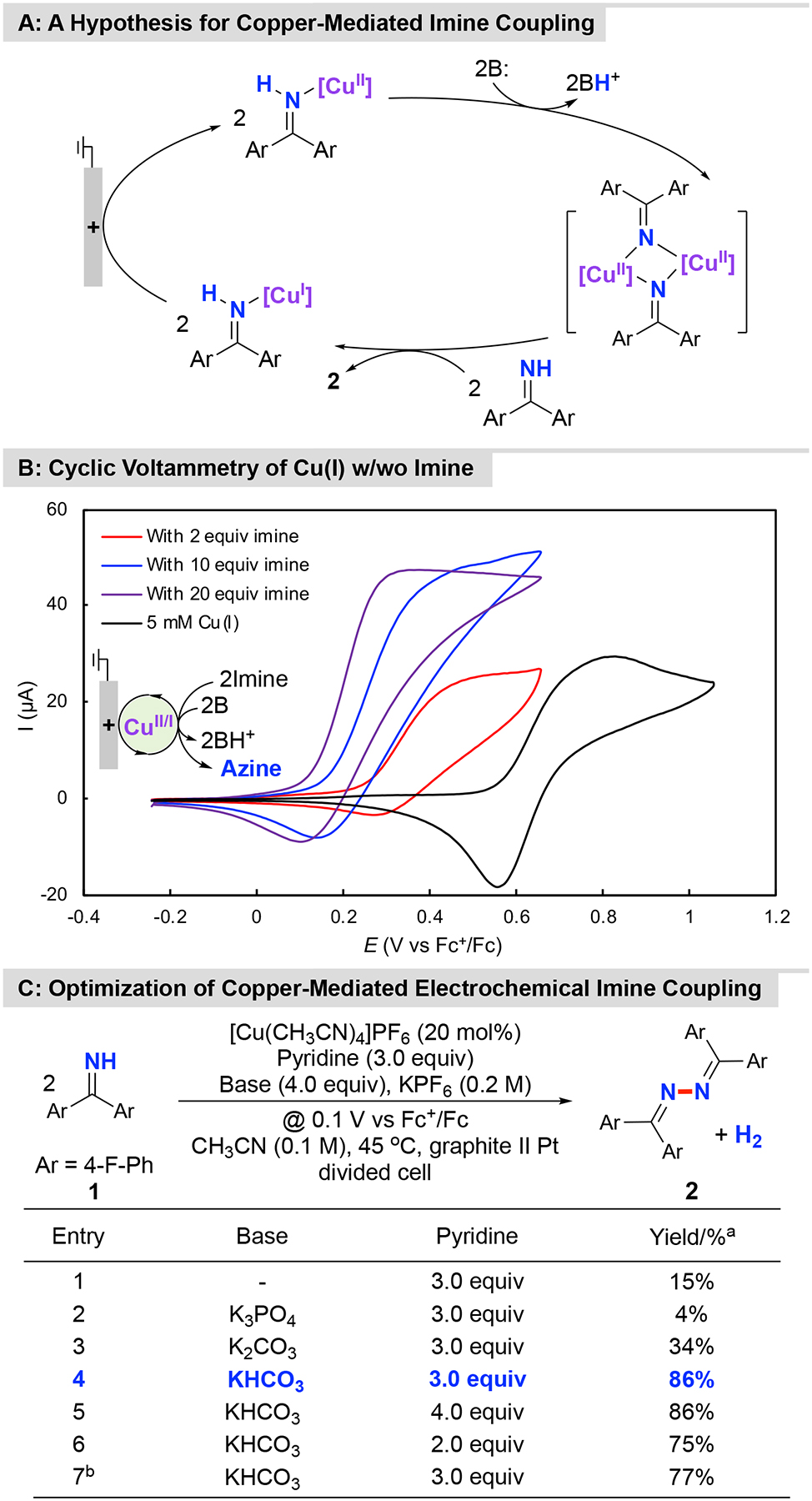
A: A hypothesis for copper-mediated imine coupling; B: Cyclic voltammetry of [Cu(CH3CN)4]PF6 w/wo imine, conditions: 5 mM [Cu(CH3CN)4]PF6 in CH3CN (10 mL) with nBu4NPF6 (0.1 M) as supporting electrolyte, with glassy carbon as working electrode (~ 7.0 mm2) and a platinum wire (1.0 cm, spiral wire) as counter electrode, scan rate = 20 mV/s; C: Optimization of a copper-mediated electrochemical imine coupling. aYields were determined by 19F NMR analysis with α,α,α-trifluorotoluene as internal standard; bUse of [Cu(CH3CN)4]PF6 (10 mol%) as mediator.
