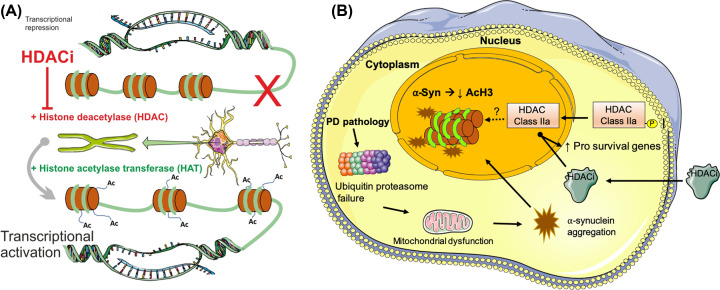
Figure 1. Class II HDAC inhibitors as potential neuroprotective agents for PD therapy.
(A) Histone acetylation is a common epigenetic mechanism and is mediated by histone acetyltransferases (HAT) enzymes, which acetylate (Ac) lysine residues in the N-terminal of histone proteins. This neutralises the positive charge on the histone tail, decreasing DNA–histone interactions, which renders the DNA more accessible for transcription factor binding and thus enhances gene expression (transcriptional activation). Histone deacetylation is the opposite process and is mediated histone deacetylases (HDACs) enzymes, which remove acetyl groups, which renders the DNA less accessible for transcription factor binding, thus inhibiting gene expression (transcriptional repression). HDAC inhibitors (HDACi) are a novel class of drugs that inhibit the activity of HDACs, increasing acetylation of lysine residues on histone proteins, thus inhibiting transcriptional repression (indicated by the red X) and enhancing transcriptional activation. (B) In PD, intracellular events, including failure of the ubiquitin proteasome system and mitochondrial dysfunction, culminate in the accumulation of α-synuclein aggregates in neurons. This results in the impairment of histone acetylation and the translocation of Class IIa HDACs from the cytoplasm to the nucleus. Treatment with inhibitors of Class IIa HDACs (HDACi) can reverse these effects and increase levels of pro-survival genes, supporting a role for Class IIa HDACi as neuroprotective therapies for PD.