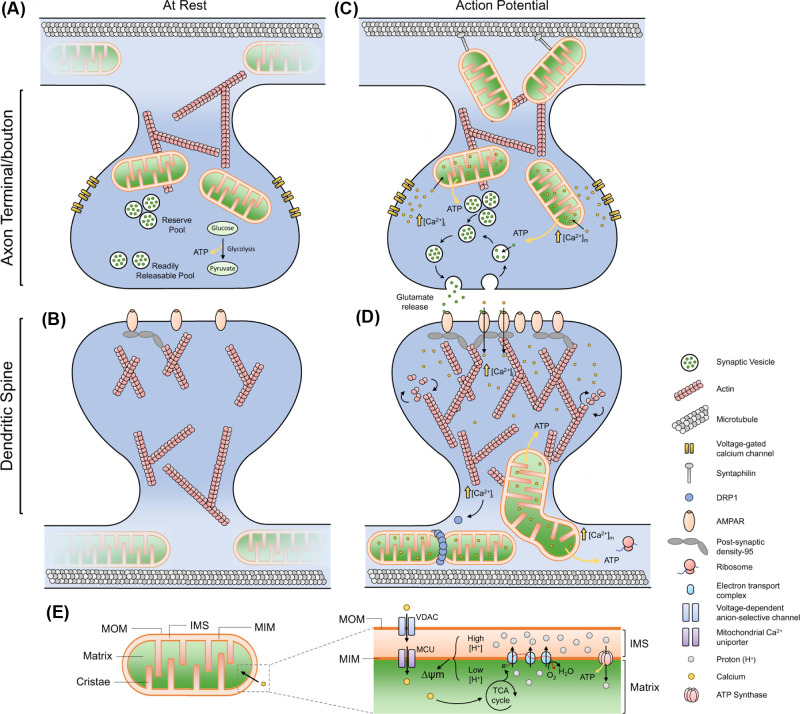
Figure 2. Roles of mitochondrial dynamics and calcium in support of neuronal activity and plasticity.
(A) Axonal mitochondria are very dynamic, exhibiting both anterograde and retrograde movement. At the presynapse, glycolysis and OXPHOS support basal activity of the synapse. (B) Dendritic mitochondria are larger than axonal mitochondria and exhibit more stabilised behaviour, with a small number of spines containing mitochondria. (C) During an action potential, an influx of Ca2+ rapidly increases cytoplasmic [Ca2+], also increasing mitochondria [Ca2+], which stimulates OXPHOS activity and increased ATP generation. The enhanced ATP generation powers energy demanding processes such as vesicle recycling, endocytosis and mobilisation of the reserve pool. Local mitochondria stop trafficking and are stabilised by the action of syntaphilin (anchoring protein) and rearrangements of the Miro-TRAK complex. Axonal mitochondria also buffer Ca2+ to prevent sustained elevations of [Ca2+]i, thus regulating continuous rounds of firing, while also preventing asynchronous transmission. (D) Ca2+ influx at the postsynapse causes dendritic mitochondria to protrude into the spine, and also causes mitochondrial fission, which regulates Ca2+ transients in the mitochondria during activity. Dendritic mitochondria provide ATP for actin-dynamics, supporting growth of the postsynaptic density, surface expression of neurotransmitter receptors and supports local translation. (E) Schematic of mitochondrial compartments and Ca2+ influx. Mitochondria consist of two membranes: the mitochondrial outer membrane (MOM) and the mitochondrial inner membrane (MIM). The region formed between the MOM and MIM is called the intermembrane space (IMS). The MIM has extensive folds, which form the cristae, increasing the surface area of the membrane which houses the electron transport chain (ETC) components. Substrates from the tricarboxylic acid (TCA) cycle act as electron donors to the ETC, which couples electron transfer with the shuttling of protons across the MIM into the IMS, forming a higher concentration of protons in the IMS compared to the matrix. This generates a concentration gradient and an electrical potential (due to charge separation), termed the mitochondrial membrane potential (∆ψm). The ∆ψm is the driving force for ATP synthesis, as protons pass down their concentration and electrical gradient, passing through the enzyme ATP Synthase, forming ATP. ∆ψm is also a driving force for Ca2+ sequestration in the matrix. Ca2+ must traverse both the MOM and MIM to enter the matrix. The voltage-dependent anion-selective channel (VDAC) and the mitochondrial Ca2+ uniporter (MCU) make the MOM and MIM permeable to Ca2+, respectively. Matrix localised Ca2+ stimulates the TCA cycle and enhances mitochondrial OXPHOS function.