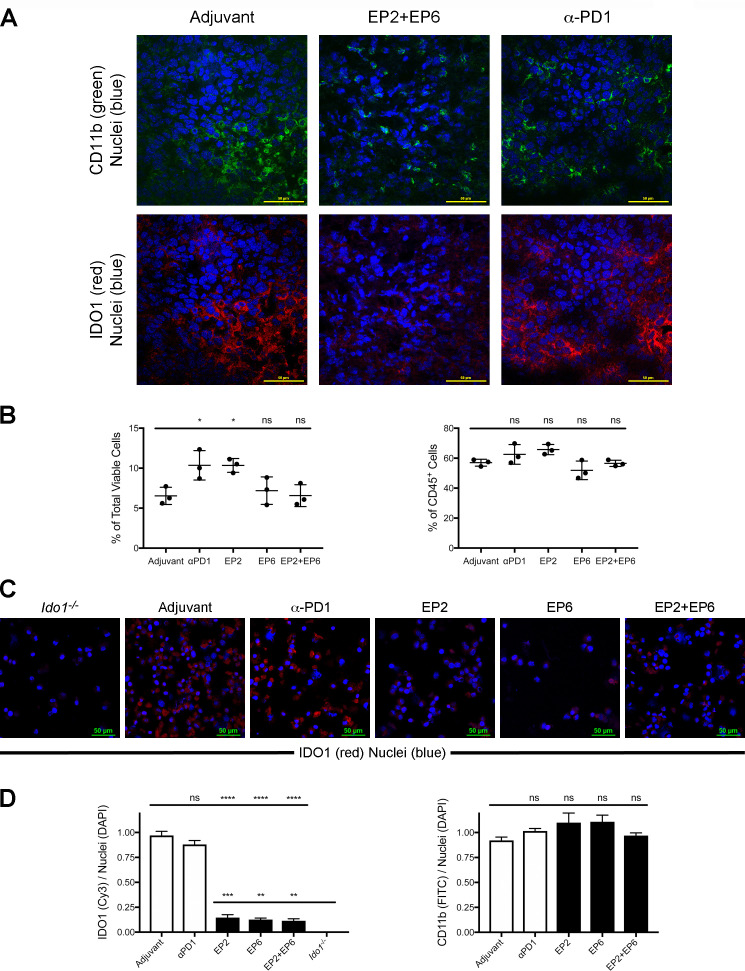
Figure 5.
Major histocompatibility complex (MHC) class I-directed and II-directed indoleamine 2,3-dioxygenase 1 (IDO1) peptide administration does not reduce the proportional representation of IDO1-expressing tumor infiltrating immune cells but does reduce their expression of IDO1. (A) Confocal images of CT26 tumor sections from wild-type (WT) mice administered either adjuvant alone, EP2+EP6 IDO1 peptides or antiprogrammed cell death protein 1 (anti-PD1) antibody as noted. Immunofluoresence staining for CD11b (fluorescein isothiocyanate (FITC), green), IDO1 (Cy3, red) and nuclei (DAPI, blue) was performed on all sections. (B) Quantitative comparison of the proportional representation of the CD11bhi CD11chi (IDO1-expressing) subset following administration of adjuvant alone, anti-PD1 and the individual EP2 or EP6 peptides alone or combined identified by flow cytometry as shown in online supplementary additional file 8 from (left) within the total population of viable cells from dissociated tumors and (right) within the CD45+ population of tumor-infiltrating immune cells. Graphed as means±SEM with significance determined by one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test. (C) Confocal images of a field of FACS-isolated CD45+, CD11b+, CD11c+ cells from CT26 tumors obtained from an Ido1-/- host or from WT hosts administered either adjuvant alone, EP2, EP6 or EP2+EP6 IDO1 peptides, or anti-PD1 antibody as noted. Immunofluoresence staining for IDO1 (Cy3, red) and nuclei (DAPI, blue) was evaluated on all sections. (D) Quantitative comparison of IDO1 (Cy3)/nuclei (DAPI) (left) and CD11b (FITC)/nuclei (DAPI) (right) staining per field from FACS-isolated CD45+, CD11b+, CD11c+ cells from treatment groups described in (D). Graphed as means±SEM with significance determined by one-way ANOVA with Tukey’s multiple comparison test. ns, not significant. *P<0.05;**p<0.01; ***p<0.001, ****p<0.0001.