Figure 2.
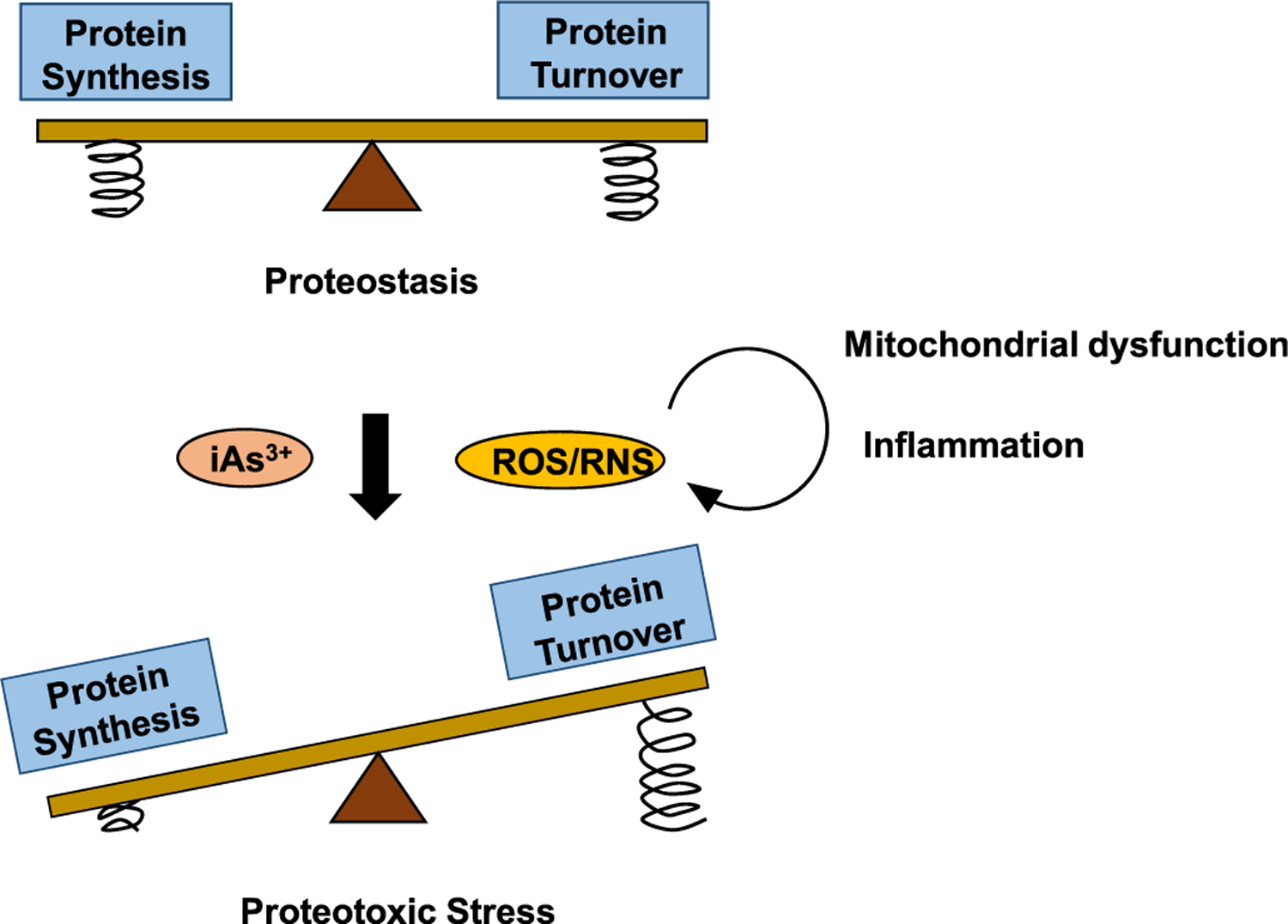
Proteostasis is achieved by sustaining a balance between protein synthesis and protein turnover, with arsenic exposure and arsenic-elicited overproduction of ROS/RNS tipping the equilibrium away through impairing the degradative capacity of the proteostasis network. Arsenic exposure diminishes the folding capacity of molecular chaperones, resulting in the increased formation of misfolded/aggregated proteins. In addition, arsenic exposure and ROS/RNS impair the UPS, autophagy, and asymmetric segregation and axonal transport of damaged proteins, reducing the degradative capacity of the proteostasis network. Mitochondrial dysfunction and inflammation induced by arsenic exposure form a vicious self-feeding cycle of excessive ROS/RNS production, further aggravating proteotoxic stress through the imbalance between protein synthesis and turnover. Compression spring in the image represents the feedback mechanisms regulating the PQC machineries of protein synthesis or those of protein turnover. Arsenic and ROS/RNS can perturb the feedback signaling pathways involved in the PQC.
