Abstract
Objective
Coronavirus disease 2019 (COVID-19) has become a global pandemic and may adversely affect pregnancy outcomes. We estimated the adverse maternal and neonatal characteristics and outcomes among COVID-19 infected women and determined heterogeneity in the estimates and associated factors.
Study Designs
PubMed search was performed of confirmed COVID-19 pregnant cases and related outcomes were ascertained prior to July 8, 2020, in this systematic review and meta-analysis. Studies reporting premature birth, low birth weight, COVID-19 infection in neonates, or mode of delivery status were included in the study. Two investigators independently performed searches, assessed quality of eligible studies as per the Cochrane handbook recommendations, extracted and reported data according to PRISMA guidelines. Pooled proportions of maternal and neonatal outcomes were estimated using meta-analyses for studies with varying sample sizes while a systematic review with descriptive data analysis was performed for case report studies. Maternal and neonatal outcomes included C-section, premature birth, low birth weight, adverse pregnancy events and COVID transmission in neonates.
Results
A total of 790 COVID-19 positive females and 548 neonates from 61 studies were analyzed. The rates of C-section, premature birth, low birth weight, and adverse pregnancy events were estimated as 72 %, 23 %, 7 %, and 27 % respectively. In the heterogeneity analysis, the rate of C-section was substantially higher in Chinese studies (91 %) compared to the US (40 %) or European (38 %) studies. The rates of preterm birth and adverse pregnancy events were also lowest in the US studies (12 %, 15 %) compared to Chinese (17 %, 21 %), and European studies (19 %, 19 %). In case reports, the rates of C-section, preterm birth, and low birth weight were estimated as 69 %, 56 %, and 35 %, respectively. Adverse pregnancy outcomes were associated with infection acquired at early gestational ages, more symptomatic presentation, myalgia symptom at presentation, and use of oxygen support therapy.
Conclusions
Adverse pregnancy outcomes were prevalent in COVID-19 infected females and varied by location, type, and size of the studies. Regular screening and early detection of COVID-19 in pregnant women may provide more favorable outcomes.
Keywords: COVID-19, Preterm birth, Low birth weight, Adverse pregnancy outcomes, Meta-analysis, Cesarean section
Introduction
The coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and has now become a global pandemic rapidly growing across the world [1]. It has become critical to identify at-risk individuals for COVID-19 to avoid poor outcomes through early, aggressive, and preventive management [2]. Although COVID-19 can affect anyone, pregnant women may be more susceptible to this viral infection due to physiological and immunological changes during pregnancy [3]. One of the major consequences of viral pneumonia is deaths during pregnancy worldwide [4]. Moreover, viral infections have been associated with adverse pregnancy and neonatal outcomes [5]. Therefore, there is a concern of having adverse pregnancy outcomes due to intrauterine transmission of infection to fetus from mother [6,7]. Studies have shown the impact of viral infections on preterm births and related outcomes [8]. Premature births also have long-term consequences of developmental delays. However, the current burden of adverse pregnancy events including preterm birth and low birth weight is unclear among females infected with SARS-CoV-2 particularly in comparison with women without infection.
Recently, a few narrative and some systematic reviews based on limited studies have attempted to summarize the maternal and neonatal characteristics of pregnant women with COVID-19 [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]]. Most of these studies were predominantly based on case reports, conducted a qualitative analysis by combining mixed studies of case series with case reports, and are not up-to-date. Furthermore, none of these studies estimated the overall adverse pregnancy outcomes comprising preterm birth, death/stillbirth, and early termination of pregnancies. Although studies evaluating the effect of COVID-19 infection on pregnancy outcomes have been accumulating, the maternal characteristics and treatment profile for managing COVID-19 and pregnancy outcomes were inconsistent across the studies. Because of the mixing of case reports with case-series and not utilizing appropriate statistical analyses, previous studies yielded biased estimates of a mode of delivery and pregnancy outcomes. There is a need to determine heterogeneity in the estimates of adverse pregnancy outcomes and factors associated with adverse pregnancy outcomes separately for preterm birth, adverse pregnancy events, and low birth weight. To promote evidence-based practice among infected pregnant women, it is vital to provide quantitative syntheses of adverse pregnancy outcomes to health care providers timely and accurately. We sought to estimate the proportions of adverse pregnancy outcomes along with premature birth, low birth weight, and C-section rates among mothers with COVID-19 through an updated systematic review and meta-analysis of case series and case reports separately.
Materials and methods
Search methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines to perform this study [24,25]. A comprehensive search on PubMed was made for any studies reporting data on pregnant women with COVID-19 prior to July 8th, 2020. We used combinations of search terms “COVID-19 OR SARS-CoV-2 OR Coronavirus” AND “Pregnancy OR Pregnant” OR “Neonates OR Newborn OR Neonatal” to screen all eligible articles. Full articles and abstracts were independently reviewed for evaluating eligibility criteria by the two authors (PD and AD). Review and duplicated articles were excluded from the analysis. References from the review articles were also cross-checked to screen any pertinent studies excluded from the initial search. Efforts were made to avoid studies with overlapping results or inclusion of case data. We double-checked all the eligible articles for any duplicity of data reporting in two or more articles by matching the period of patient recruitment, location, or author of studies, and excluded from our study.
Inclusion and exclusion criteria
Only studies were included in which patients were pregnant on admission, confirmed with a COVID-19 infection by quantitative real-time polymerase chain reaction (qRT-PCR), reported data on human subjects with primary preterm birth, low birth outcome or mode of delivery, and written in English or could be translated into English from the Chinese language. Studies with unpublished reports, unconfirmed COVID-19 cases and without maternal or neonatal outcomes, and clinical trials were excluded from the study.
Study endpoints
We used a comprehensive data sheet to extract data on the following variables a) neonatal outcomes which included the number of neonates, type of delivery (C-section or normal), low birth weight, infant death or stillbirth, neonates with COVID-19 infection, asphyxia, pneumonia, lymphopenia, and Apgar score b) maternal characteristics which included age range, gestation age, comorbidities (gestational diabetes, pre-eclampsia, and others), and clinical symptoms (fever, cough, dyspnea, fatigue, diarrhea, sore throat, myalgia, lymphopenia before and after treatment, elevated C-reactive protein, and asymptomatic) and outcomes (preterm birth, death, and early pregnacy termination) c) treatment profile (oxygen support, antivirals and antibiotics). The primary outcomes of interest in the study were C-section rate, rate of premature birth, rate of low birth weight, overall adverse pregnancy events (defined as a composition of premature birth and stillbirth or death or early pregnancy termination), and the rate of COVID infection in neonates. In addition to these data, we also extracted the type of study (case reports or case series). Any studies with less than 5 cases were classified into a case report. The location of the study was classified into China, USA, Europe (Ireland, Italy, Netherlands, Spain, Sweden, Turkey, and UK). Rest studies were classified into others category (Jordan, Iran, Korea, India, Peru, Canada, and Australia) due to a low number of case studies. The number of pregnant women with a positive COVID infection, number of women proceeded to delivery or still pregnant, number of women with pregnancy outcome status, and the number of neonates were also extracted and used for estimating specific proportions.
Assessment of risk of bias
We performed the quality appraisal of eligible studies. The risk of bias was assessed using the quality assessment tool for case series studies (NHLBI, Research Triangle Institute International. National Heart, Lung, and Blood Institute Quality Appraisal Tools) [26]. In addition, funnel plots and Egger’s test were performed to assess publication bias as well.
Statistical analysis
We performed (a) a meta-analysis for primary outcomes (C-section rates, preterm birth rates, low birth weight rates, adverse pregnancy events, common symptoms, common treatment) after assigning appropriate weight to each study (b) descriptive data analyses for these outcomes in case reports studies without assigning any weight. Studies with 5 or more subjects were considered in the meta-analysis. The pooled proportions (P) were also estimated separately for location and study size. Appropriate summary measures such as mean and range were used for quantitative measures while frequency and proportions were used for categorical data in the descriptive data analysis [27]. For the meta-analysis, we applied a priori random effects model for proportions using the DerSimonian and Laird (D-L) method. The Wilxon score method was used to obtain a 95 % confidence interval (CI) for the pooled estimate. The pooled association between pregnancy outcomes and COVID-19 compared to controls was summarized with an odds ratio (OR) and a 95 %CI. The I2 statistic was used to summarize the proportion of observed variance after removing sampling error to assess the heterogeneity across the studies. The I2 statistic >50 % indicates a significant presence of heterogeneity. Sensitivity and subgroup analyses according to some important cofactors were performed to evaluate heterogeneity across the studies as well as to identify factors associated with adverse pregnancy outcomes. Fishers’ exact test was conducted to determine factors associated with adverse pregnancy outcomes in case reports analysis. Forest plots were made to show key findings in the study. P-values less than 5 % were considered significant. All statistical analyses were employed using STATA 15.1 (StataCorp LLC, College Station, TX).
Results
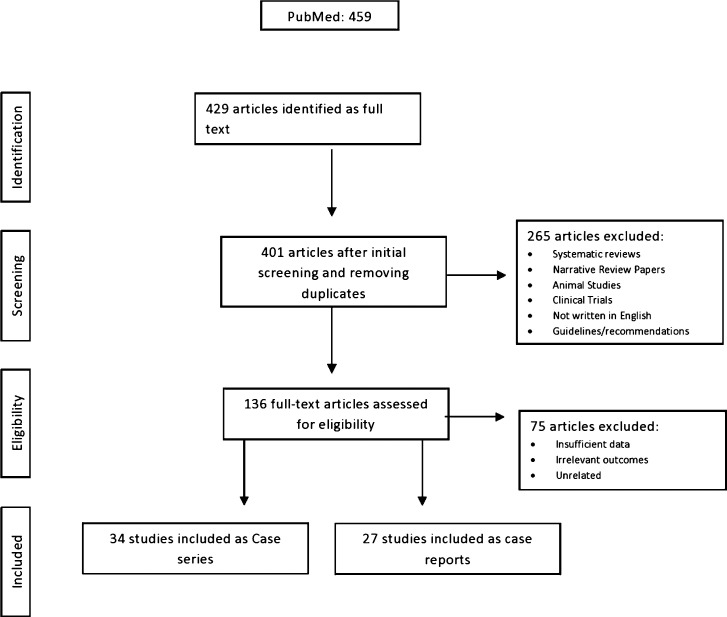
A total of 429 studies were evaluated and 61 met the eligibility criteria for data extraction and data analysis. Of these, 27 studies were used in meta-analysis and underwent for quality assessment and bias evaluation (Fig. 1 ). The list of excluded studies along with PubMed identifier and pertaining reasons for exclusion are included in Supplementary Table 1. Accordingly, 11 clinical trial studies, 7 demographic studies, 12 immunological or pathological studies, 35 studies with insufficient data, 5 clinical updates or commentaries, and 3 maternal health studies were excluded. In addition, 2 studies were excluded due to duplicity of data reporting. Out of 27 studies [[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43]], 16 studies were from China (14 were from Hubei province and 2 from Guangzhou) and 4 studies was from USA [[44], [45], [46], [47]], 2 from France [48,49], 2 from Italy [50,51], 1 from UK [52], 1 from Spain [53], and 1 from Iran [54]. Of 27 studies, 4 studies (n = 233) also included a control group [[38], [39], [40], [41]]. However, one study included non-pregnant healthy controls [39]. The meta-analysis was based on 745 COVID infected cases and 219 controls. A total of 34 case reports [[55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88]] yielding 45 cases were also included in the descriptive data analysis. Majority of the studies (12/34) were from China, 7 from the USA [62,67,68,70,76,85,87], 2 from Iran [63,80], 2 from Netherlands [82,83], 1 from Peru [75], 1 from Italy 1 [84], 1 from Sweden [64], 1 from Spain [73], 1 from Korea [55], 1 from Turkey [56], 1 from UK [78], 1 from Australia [88], 1 from Canada [79], 1 from Jordon [86], and 1 from India [72].
Fig. 1.
Study flowchart. Inclusion of studies at different stages for this systematic review and meta-analysis.
Maternal and neonatal characteristics
The average maternal age of women included in the meta-analysis was 31 (midrange: 28−37) years with an average gestational week of 34.3 (midrange: 25−39.5). The majority of patients had no lymphopenia after treatments. A total of 505 neonatal subjects were included in the meta-analysis. Only 6 studies reported a COVID infection in neonates and 8 studies reported other events such as death or stillbirth in neonates (Table 1 ).
Table 1.
Characteristics of studies included in the analysis.
| Study | Place | Women with COVID | Neonates | Average midrange age (range), y | Average midrange gestation (range), w | Cesarean section | Adverse events | Preterm birth | Death/stillbirth/early termination | Low birth weight | Newborn with COVID-19 | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CASE SERIES STUDIES INCUDED IN THE META-ANALYSIS | ||||||||||||
| Liu et al [28] | China | 15 | 11 | 31.5(23−40) | 25(12−38) | 10 | 3 | 3 | 0 | NR | 0 | Good |
| Chen et al [29] | China | 9 | 9 | 33(26−40) | 37.1(36−39) | 9 | 4 | 4 | 0 | 2 | 0 | Good |
| Chen et al [30] | China | 5 | 5 | 28(25−31) | 39.5(38−41) | 3 | 0 | 0 | 0 | 0 | 0 | Good |
| Yu et al [31] | China | 7 | 7 | 31.5(29–34) | 39(37−41) | 7 | 0 | 0 | 0 | 0 | 1 | Good |
| Wu et al [32] | China | 23 | 21 | 29(21−37) | 25.5(12−39) | 18 | 6 | 3 | 3 | 0 | 0 | Good |
| Liu et al [33] | China | 13 | 10 | 29.5(22−37) | 32(25−39) | 10 | 7 | 6 | 1 | NR | NR | Fair |
| Zhu et al [34] | China | 9 | 10 | 30(30) | 35(31−39) | 9 | 7 | 6 | 1 | 7 | NR | Fair |
| Khan et al [35] | China | 17 | 17 | 29(24−34) | 38(35−41) | 17 | 3 | 3 | 0 | 3 | 0 | Fair |
| Breslin et al [44] | USA | 43 | 18 | 29.5(20−39) | 35(32−38) | 8 | 1 | 1 | 0 | NR | NR | Fair |
| Liu et al [36] | China | 19 | 19 | 31(26−36) | 38(35−41) | 18 | 2 | 2 | 0 | 1 | 0 | Good |
| Zeng et al [37] | China | NR | 33 | NR | NR | NR | 4 | 4 | 0 | 2 | 3 | Fair |
| Zhang et al [38] * | China | 16 | 11 | 29(24−34) | 38(35−41) | 10 | 8 | 3 | 5 | 0 | 0 | Good |
| Liu et al [39] * | China | 41 | 16 | 30(30) | NR | NR | NR | NR | NR | NR | 0 | Fair |
| Li et al [40] * | China | 16 | 17 | 31.5(26−37) | 36.5(33−40) | 14 | 4 | 4 | 0 | 3 | 0 | Good |
| Hantoushzadeh et al [54] $ | Iran | 9 | 11 | 37(25−49) | 31(24−38) | 6 | 8 | 8 | 5 | 7 | 0 | Good |
| Liao et al [41] * | China | 10 | 10 | 31.5(27−36) | 36.5(31−42) | 0 | 1 | 1 | 0 | 0 | 0 | Good |
| Ferrazzi et al [50] | Italy | 42 | 40 | 32.9(21−43) | NR | 18 | 11 | 11 | 0 | 3 | Good | |
| Govind [52] | UK | 9 | 9 | 28.5(18−39) | 36.7(27−39) | 8 | 2 | 2 | 0 | 2 | 1 | Fair |
| Wu et al [42] | China | 8 | 8 | 29.8(26−35) | 37.7(33−40) | 6 | NR | NR | NR | NR | NR | Fair |
| Pereira et al [53] | Spain | 60 | 23 | 34 (22−43) | 32(5−41) | 5 | 2 | 2 | 0 | NR | 0 | Good |
| Sentilhes et al [48] | France | 54 | 21 | 30.6 | 30.4 | 9 | 3 | 3 | 0 | 0 | 0 | Fair |
| Blitz [45] | USA | 13 | 7 | 33.8 | 33.3 | 6 | 7 | 5 | 2 | 0 | NR | Fair |
| Savasi et al [51] + | Italy | 77 | 57 | 32 (15−48) | 37.2(5−41) | 22 | 12 | 12 | 0 | NR | 4 | Fair |
| London et al [46] | USA | 68 | 55 | 30(24.5−34.8) | NR | 22 | 10 | 9 | 1 | NR | 0 | Good |
| Zeng et al [43] | China | 16 | 16 | 31(25−40) | 37(34−41) | 12 | 3 | 3 | 0 | 0 | 0 | Fair |
| Lokken et al [47] ++ | USA | 46 | 8 | 29(26−34) | 27(21−33.9) | 3 | 2 | 1 | 1 | 0 | 0 | Fair |
| Vivanti et al [49] | France | 100 | 36 | 33.7(29−36.7) | 31.3(25.6−35.6) | 16 | 20 | 20 | 0 | 0 | 1 | Good |
| Total(27) | 745 | 505 | 31.0(28−37) | 34.3(25−39.5) | 266 | 130 | 116 | 19 | 27 | 13 | ||
| CASE REPORTS (INDIVIDUAL PATIENT ANALYSIS) | ||||||||||||
| Lee et al [55] | Korea | 1 | 1 | 35 | 37 | 1 | 0 | 0 | 0 | 0 | 0 | NA |
| Kalafat et al [56] | Turkey | 1 | 1 | 32 | 35 | 1 | 0 | 0 | 0 | 0 | 0 | NA |
| Yang et al [57] | China | 1 | 1 | 30 | 35 | 1 | 1 | 1 | 0 | NR | 0 | NA |
| Fan et al [58] | China | 2 | 2 | 31.5 | 36.5 | 2 | 1 | 1 | 0 | 0 | 0 | NA |
| Wang et al [59] | China | 1 | 1 | 34 | 40 | 1 | 0 | 0 | 0 | 0 | 1 | NA |
| Zambrano et al [60] | China | 1 | 1 | 41 | 32 | 0 | 1 | 1 | 0 | 1 | 0 | NA |
| Wang et al [61] | China | 1 | 1 | 28 | 30 | 1 | 1 | 1 | 0 | 1 | 0 | NA |
| Iqbal et al [62] | USA | 1 | 1 | 34 | 39 | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Karami et al [63] | Iran | 1 | 1 | 27 | 30 | 0 | 2 | 1 | 1 | NA | 1 | NA |
| Gidlöf et al [64] | Sweden | 1 | 2 | 34 | 36 | 1 | 2 | 2 | 0 | 2 | 0 | NA |
| Chen et al [65] | China | 4 | 4 | 28.5 | 37 | 3 | 0 | 0 | 0 | 0 | 0 | NA |
| Chen et al [66] | China | 3 | 3 | 29.5 | 36.5 | 3 | 1 | 1 | 0 | 1 | 0 | NA |
| Blauvelt et al [67] | USA | 1 | 1 | 34 | 28 | 1 | 1 | 1 | 0 | 1 | 0 | NA |
| Hong et al [68] | USA | 1 | 0 | 36 | 23 | 0 | NR | NR | NR | NR | NR | NA |
| Li et al [69] | China | 1 | 1 | 31 | 35 | 1 | 2 | 1 | 1 | 0 | 0 | NA |
| Browne et al [70] | USA | 1 | 0 | 33 | 27 | 0 | NR | NR | NR | NR | NR | NA |
| Lu et al [71] | China | 1 | 1 | 22 | 38 | 1 | 0 | 0 | 0 | 0 | 0 | NA |
| Sharma et al [72] | India | 1 | 1 | NR | 36 | 1 | NR | NR | NR | NR | NR | NA |
| Romero et al [73] | Spain | 1 | 1 | 44 | 29 | 1 | 1 | 1 | 0 | NR | 0 | NA |
| Peng et al [74] | China | 1 | 1 | 25 | 35 | 1 | 1 | 1 | 0 | 1 | 0 | NA |
| Alzamora et al [75] | Peru | 1 | 1 | 41 | 33 | 1 | 1 | 1 | 0 | 0 | 1 | NA |
| Mehta et al [76] | USA | 1 | 2 | 39 | 27 | 1 | 0 | 2 | 0 | 2 | 1 | NA |
| Yu et al [77] | China | 1 | 1 | 35 | 34 | 0 | 1 | 1 | 0 | 1 | 0 | NA |
| Cooke et al [78] | UK | 2 | 2 | 30.5 | 28 | 2 | 2 | 2 | 0 | 2 | 0 | NA |
| Kirtsman et al [79] | Canada | 1 | 1 | 40 | 35 | 1 | 1 | 1 | 0 | 0 | 1 | NA |
| Taghizadieh et al [80] | Iran | 1 | 1 | 33 | 34 | 1 | 1 | 1 | 0 | 0 | 0 | NA |
| Khan et al [81] | China | 3 | 3 | 32 | 37 | 0 | 1 | 1 | 0 | 0 | 0 | NA |
| Fontanella et al [82] | Netherlands | 2 | 1 | 33.5 | 35.5 | 1 | 0 | 0 | 0 | 0 | 1 | NA |
| Grimminck et al [83] | Netherlands | 1 | 1 | 31 | 38 | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Ferraiolo et al [84] | Italy | 1 | 1 | 30 | 38 | 1 | 0 | 0 | 0 | 0 | 0 | NA |
| Silverstein et al [85] | USA | 2 | 2 | 25.5 | 35 | 2 | 2 | 2 | 0 | 1 | 0 | NA |
| AlZaghal et al [86] | Jordan | 1 | 1 | 30 | 36 | 1 | 1 | 1 | 0 | 1 | 0 | NA |
| Anderson et al [87] | USA | 1 | 0 | 35 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Lowe et al [88] | Australia | 1 | 1 | 31 | 40 | 0 | 0 | 0 | 0 | 0 | 0 | NA |
| Total (34) | 45 | 43 | 32.6 | 33.8 | 31 | 22 | 24 | 2 | 14 | 6 | NA | |
Included a control group in the study; w: weeks; y: years: midrange is the average of the range reported; NR: not reported; NA: not applicable.
5 adverse events of 8 preterm births.
status is missing for 1 patient.
consent was not obtained for 4 patients.
For the case reports, the average age was 32.6 (range: 22–44) years with an average gestational week of 33.8 (range: 22–40). For single case studies, 43 neonates were included in the analysis (Table 1). Among reported studies, all neonates had a 5-minute Apgar score of 8 or higher except four cases with a 0, 1, 3, and 6 score. Of 34 case reports, 6 studies observed infection in neonates and two studies reported death or stillbirth in neonates (Table 1).
Meta-analysis
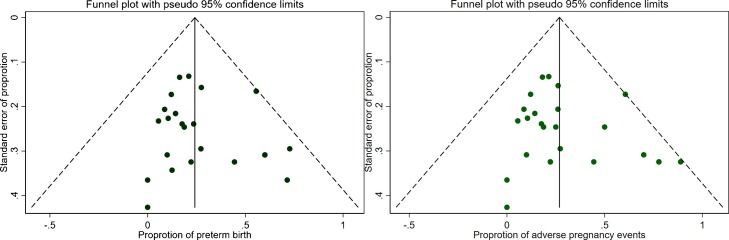
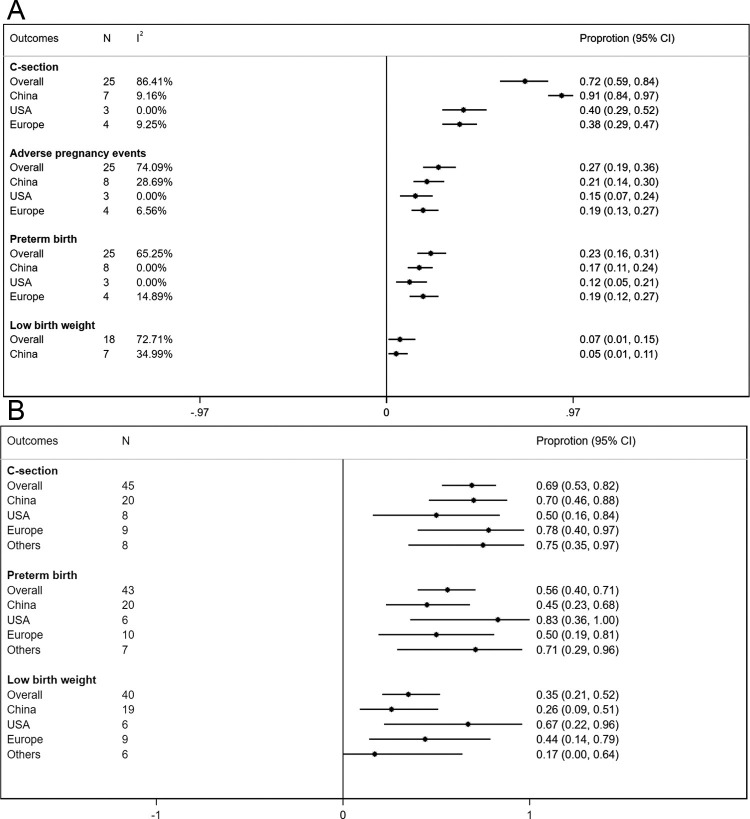
Most of the studies included in the meta-analysis were of fair or good quality (Table 1). The symmetry of the funnel plots (Fig. 2 A and B) and Egger’s test p-values (p = 0.44 for preterm birth and p = 0.23 for adverse pregnancy events) indicated no significant presence of publication bias or small size effect. Of the 745 pregnant women, 474 subjects proceeded to delivery and 8 had early abortions, while 263 were still pregnant at the end of the study. The pooled rate of C- section was commonly observed as 72 % (95 %CI: 0.59−0.84, I2 = 86.4 %). Of the total successful births, the pooled proportions were estimated to be 23 % (95 %CI: 0.16−0.31, I2 = 65.3 %) for preterm birth and 7 % (95 %CI: 0.01−0.15, I2 = 72.7 %) for low birth weight. Among 509 females, 27 % (95 %CI: 0.19−0.36, I2 = 74.1 %) had overall adverse pregnancy events (Fig. 3 A). Compared to controls, the odds of preterm birth was higher (n = 3, OR = 2.28; 95 %CI: 0.92–5.65, p = 0.074) in COVID-19 cases.
Fig. 2.
(A) Funnel plot for preterm birth outcome; (B) Funnel plot for adverse pregnancy events.
Fig. 3.
(A) Rates of cesarean section, adverse pregnancy events, preterm birth, and low birth weight estimated from case series studies. The meta-analysis findings of case series studies reported separately for Chinese studies with a sample size>10, the USA studies after excluding a study with low sample size, and European studies after excluding one study with small sample size and another study with a large number of women without pregnancy status. N denotes the number of datasets or cases, I2 denotes the measure of heterogeneity across studies. The adverse pregnancy events include preterm birth, death, stillbirth, and early terminated pregnancies; (B) Rates of cesarean section, preterm birth, and low birth weight estimated from case report studies by location of the study. European studies included studies from Ireland, Italy, Netherlands, Spain, Sweden, Turkey, and UK; others included studies from countries (Jordan, Iran, Korea, India, Peru, Canada, and Australia).
For pregnant subjects, the most prevalent symptom was fever (58 %), followed by cough (52 %), lymphopenia (46 %), and dyspnea (17 %). The asymptomatic presentation was estimated to be 9% in COVID infected patients. The elevated C-reactive protein (CRP) was estimated to be 48 % of infected patients. From studies reporting the treatment profile, the most common treatment was antibiotics (67 %), followed by antiviral medications (48 %), and oxygen support (31 %). Only limited studies reported comorbidities. Among reported comorbidities, other types of comorbidities (such as polycystic ovary syndrome and hypothyroidism) were the most common (13 %) followed by preeclampsia (4 %) and gestational diabetes (4 %) (Table 2 ).
Table 2.
Symptoms and treatments profile.
| Meta-analysis of case series studies |
Descriptive data analysis of case reports | |||||
|---|---|---|---|---|---|---|
| Outcomes | Meta-analysis |
Individual data analysis | ||||
| N | I2 | P(%) | 95 %CI | P(%) | ||
| Maternal signs and symptoms | ||||||
| Fever | 26 | 83.67 % | 0.58 | 0.48 | 0.67 | 0.64 |
| Cough | 26 | 85.49 % | 0.52 | 0.42 | 0.62 | 0.60 |
| Lymphopenia | 18 | 91.40 % | 0.46 | 0.31 | 0.62 | 0.38 |
| Asymptomatic | 20 | 84.94 % | 0.09 | 0.02 | 0.19 | 0.09 |
| Dyspnea | 25 | 89.11 % | 0.17 | 0.09 | 0.28 | 0.36 |
| Fatigue | 21 | 77.16 % | 0.06 | 0.01 | 0.12 | 0.16 |
| Diarrhea | 23 | 48.86 % | 0.03 | 0.01 | 0.06 | 0.04 |
| Sore throat | 22 | 66.57 % | 0.03 | 0.01 | 0.08 | 0.22 |
| Myalgia | 21 | 83.25 % | 0.06 | 0.01 | 0.14 | 0.24 |
| Elevated C-reactive protein | 18 | 89.58 % | 0.48 | 0.34 | 0.63 | 0.55 |
| Maternal comorbidities | ||||||
| Gestational diabetes | 20 | 39.69 % | 0.04 | 0.02 | 0.07 | 0.21 |
| Preeclampsia | 20 | 25.56 % | 0.04 | 0.02 | 0.07 | 0.08 |
| Other* | 20 | 63.71 % | 0.13 | 0.08 | 0.2 | 0.44 |
| Treatments for COVID-19 infection | ||||||
| Antibiotics | 17 | 96.79 % | 0.67 | 0.41 | 0.89 | 0.63 |
| Antivirals | 17 | 96.44 % | 0.48 | 0.24 | 0.73 | 0.69 |
| Oxygen support | 18 | 90.99 % | 0.31 | 0.19 | 0.46 | 0.71 |
Other included polycystic ovary syndrome, hypothyroidism, hyperthyroidism etc.; P: proportion; CI: confidence interval; I2: measures of heterogeneity across studies; COVID: coronavirus disease.
Only 13 (1 %, 95 %CI: 0 %–2 %, I2 = 0 %) neonatal subjects had a COVID-infection. A total of 19 (2 %, 95 %CI: 0 %–5 %, I2 = 53.4 %) death/stillbirth which included 5 early terminated pregnancies and 3 threatened abortions was also observed (Table 1). In newborns, pneumonia was reported in 13(1 %, 95 %CI: 0 %–3 %, I2 = 45.8 %) neonates while 4 neonates had asphyxia and lymphopenia.
Heterogeneity assessment
Location and sample size of the included studies were major contributors to heterogeneity across the studies. After stratifying studies by study location, the heterogeneity for estimating C-section rate was substantially reduced among the US studies (48 %, I2 = 43.3 %) and European studies (44 %, I2 = 62.2 %). There were no differences observed in other pregnancy outcomes (preterm birth and adverse pregnancy events) by location. However, a substantial reduction in the heterogeneity measure was observed for adverse pregnancy outcomes when the analysis was restricted to studies ascertaining pregnancy outcomes with a sample size greater than 10 (Table 3 ). In the sensitivity analysis after restricting the analysis to relatively large Chinese studies [28,32,[35], [36], [37], [38], [39], [40],43], removing a US study due to relatively a low sample size [45] and two European studies due to relatively a small sample size [52] and a large number of women without pregnancy status [49], the heterogeneity was eliminated for all pregnancy outcomes. After removing heterogeneity, the rate of C-section was substantially higher in Chinese studies (91 %, I2 = 9.2 %) compared to the US (40 %, I2 = 0 %) or European (38 %, I2 = 9.3 %) studies. The rates of preterm birth and adverse pregnancy events were also lowest in the US studies (12 %, 15 %) compared to Chinese (17 %, 21 %), and European studies (19 %, 19 %) without any significant presence of heterogeneity (Fig. 3 A).
Table 3.
Heterogeneity assessment.
| N | I2 | P(%) | 95 %CI | ||
|---|---|---|---|---|---|
| C-section | |||||
| Location | |||||
| China | 14 | 79.35 % | 0.88 | 0.73 | 0.98 |
| USA | 4 | 43.30 % | 0.48 | 0.31 | 0.65 |
| Europe | 6 | 62.22 % | 0.44 | 0.32 | 0.57 |
| Sample size | |||||
| Sample size of studies with pregnancy outcome < = 10 | 11 | 87.52 % | 0.80 | 0.54 | 0.98 |
| Sample size of studies with pregnancy outcome >10 | 14 | 83.12 % | 0.66 | 0.51 | 0.80 |
| Preterm | |||||
| Location | |||||
| China | 14 | 46.99 % | 0.20 | 0.12 | 0.30 |
| USA | 4 | 71.90 % | 0.20 | 0.04 | 0.43 |
| Europe | 6 | 74.55% | 0.24 | 0.12 | 0.39 |
| Sample size | |||||
| Sample size of studies with pregnancy outcome < = 10 | 10 | 70.12 % | 0.33 | 0.15 | 0.54 |
| Sample size of studies with pregnancy outcome >10 | 15 | 53.56 % | 0.19 | 0.13 | 0.26 |
| Adverse pregnancy events | |||||
| Location | |||||
| China | 14 | 65.47 % | 0.25 | 0.14 | 0.37 |
| USA | 4 | 80.69 % | 0.26 | 0.05 | 0.54 |
| Europe | 6 | 77.96 % | 0.25 | 0.12 | 0.41 |
| Sample size | |||||
| Sample size of studies with pregnancy outcome < = 10 | 10 | 78.23 % | 0.39 | 0.17 | 0.64 |
| Sample size of studies with pregnancy outcome >10 | 15 | 63.32 % | 0.22 | 0.15 | 0.29 |
| Low birth weight | |||||
| Location | |||||
| China | 12 | 65.86 % | 0.07 | 0.01 | 0.16 |
| USA | 2 | NA | 0.00 | 0.00 | 0.13 |
| Europe | 3 | NA | 0.02 | 0.00 | 0.15 |
| Sample size | |||||
| Sample size of studies with pregnancy outcome < = 10 | 9 | 76.18 % | 0.14 | 0.01 | 0.36 |
| Sample size of studies with pregnancy outcome >10 | 9 | 43.53 % | 0.03 | 0.00 | 0.08 |
P: proportion; CI: confidence interval; I2: measures of heterogeneity across studies; NA: not applicable.
Subgroup analysis
The average age at delivery, diarrhea, and dyspnea symptoms at presentation were not associated with premature birth, adverse pregnancy outcomes, or low birth weight. Early gestational week (≤ 35) was associated with higher proportions of adverse pregnancy events and preterm birth. Studies including women with high proportions of fever, cough, fatigue, myalgia symptoms yielded a high preterm birth as well as adverse pregnancy events. Studies with high proportions of elevated CRP and lymphopenia also yielded increased adverse pregnancy outcomes. Studies with unknown CRP status and a high proportion of sore throat symptom produced the highest low birth weight. Studies including patients with less frequent asymptomatic presentation was consistently associated with more adverse pregnancy outcomes including low birth weight. An unusually high proportion of adverse pregnancy events was noticed in studies reporting more usage of antiviral medications in infected mothers. Furthermore, high premature birth, adverse pregnancy events, and low birth weight were observed in studies with increased usage of oxygen support. (Table 4 ).
Table 4.
Estimation of adverse pregnancy outcomes according to considered factors: subgroup analyses.
| Premature birth |
Adverse pregnancy events |
Low birth weight |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| P(%) | 95 %CI | P(%) | 95 %CI | P(%) | 95 %CI | ||||
| Age (years) | |||||||||
| < = 30 | 0.20 | 0.11 | 0.32 | 0.28 | 0.15 | 0.43 | 0.10 | 0.00 | 0.30 |
| >30 | 0.26 | 0.16 | 0.37 | 0.28 | 0.16 | 0.41 | 0.05 | 0.00 | 0.16 |
| Gestational age(weeks) | |||||||||
| < = 35 | 0.33 | 0.17 | 0.50 | 0.40 | 0.22 | 0.60 | 0.09 | 0.00 | 0.33 |
| >35 | 0.17 | 0.12 | 0.24 | 0.19 | 0.12 | 0.28 | 0.06 | 0.01 | 0.13 |
| Fever | |||||||||
| <50 % | 0.16 | 0.11 | 0.22 | 0.18 | 0.13 | 0.24 | 0.03 | 0.00 | 0.12 |
| > = 50 % | 0.30 | 0.18 | 0.43 | 0.36 | 0.21 | 0.51 | 0.10 | 0.00 | 0.25 |
| Cough | |||||||||
| <50 % | 0.19 | 0.11 | 0.29 | 0.22 | 0.13 | 0.33 | 0.10 | 0.01 | 0.24 |
| > = 50 % | 0.29 | 0.17 | 0.43 | 0.35 | 0.20 | 0.51 | 0.04 | 0.00 | 0.18 |
| Fatigue | |||||||||
| <10 % | 0.23 | 0.13 | 0.35 | 0.27 | 0.15 | 0.42 | 0.10 | 0.00 | 0.29 |
| > = 10 % | 0.32 | 0.14 | 0.53 | 0.36 | 0.16 | 0.58 | 0.04 | 0.00 | 0.18 |
| Diarrhea | |||||||||
| <10 % | 0.27 | 0.16 | 0.40 | 0.32 | 0.19 | 0.46 | 0.03 | 0.00 | 0.16 |
| > = 10 % | 0.21 | 0.09 | 0.35 | 0.23 | 0.09 | 0.41 | 0.14 | 0.02 | 0.32 |
| Sore throat | |||||||||
| <10 % | 0.26 | 0.15 | 0.38 | 0.29 | 0.17 | 0.43 | 0.04 | 0.00 | 0.15 |
| > = 10 % | 0.26 | 0.11 | 0.45 | 0.31 | 0.12 | 0.53 | 0.19 | 0.03 | 0.42 |
| Dyspnea | |||||||||
| <10 % | 0.21 | 0.11 | 0.33 | 0.26 | 0.13 | 0.41 | 0.05 | 0.00 | 0.25 |
| > = 10 % | 0.26 | 0.16 | 0.38 | 0.30 | 0.18 | 0.44 | 0.07 | 0.00 | 0.20 |
| Myalgia | |||||||||
| <10 % | 0.18 | 0.09 | 0.27 | 0.21 | 0.11 | 0.33 | 0.06 | 0.00 | 0.21 |
| > = 10 % | 0.43 | 0.26 | 0.60 | 0.48 | 0.28 | 0.69 | 0.12 | 0.00 | 0.38 |
| CRP | |||||||||
| <50 % | 0.19 | 0.13 | 0.27 | 0.24 | 0.17 | 0.32 | 0.01 | 0.00 | 0.05 |
| > = 50 % | 0.30 | 0.13 | 0.50 | 0.33 | 0.14 | 0.56 | 0.08 | 0.00 | 0.33 |
| Unknown | 0.21 | 0.10 | 0.33 | 0.24 | 0.11 | 0.40 | 0.19 | 0.04 | 0.42 |
| Lymphopenia | |||||||||
| <50 % | 0.17 | 0.13 | 0.22 | 0.19 | 0.14 | 0.24 | 0.03 | 0.00 | 0.12 |
| > = 50 % | 0.38 | 0.18 | 0.61 | 0.43 | 0.18 | 0.69 | 0.07 | 0.00 | 0.31 |
| Unknown | 0.24 | 0.10 | 0.41 | 0.30 | 0.12 | 0.50 | 0.11 | 0.00 | 0.31 |
| Asymptomatic | |||||||||
| <10 % | 0.38 | 0.23 | 0.54 | 0.44 | 0.26 | 0.63 | 0.13 | 0.00 | 0.35 |
| > = 10 % | 0.15 | 0.09 | 0.22 | 0.18 | 0.12 | 0.25 | 0.02 | 0.00 | 0.10 |
| Unknown | 0.15 | 0.10 | 0.21 | 0.19 | 0.05 | 0.38 | 0.04 | 0.00 | 0.13 |
| Oxygen support | |||||||||
| <50 % | 0.21 | 0.13 | 0.31 | 0.23 | 0.14 | 0.34 | 0.01 | 0.00 | 0.06 |
| > = 50 % | 0.40 | 0.13 | 0.71 | 0.46 | 0.13 | 0.81 | 0.16 | 0.00 | 0.53 |
| Unknown | 0.18 | 0.09 | 0.28 | 0.24 | 0.11 | 0.40 | 0.10 | 0.01 | 0.24 |
| Antivirals | |||||||||
| <50 % | 0.20 | 0.10 | 0.31 | 0.21 | 0.11 | 0.33 | 0.02 | 0.00 | 0.09 |
| > = 50 % | 0.32 | 0.15 | 0.51 | 0.38 | 0.18 | 0.61 | 0.09 | 0.00 | 0.28 |
| Unknown | 0.21 | 0.10 | 0.34 | 0.26 | 0.12 | 0.42 | 0.10 | 0.00 | 0.32 |
| Antibiotics | |||||||||
| <50 % | 0.18 | 0.08 | 0.31 | 0.19 | 0.08 | 0.33 | 0.00 | 0.00 | 0.03 |
| > = 50 % | 0.28 | 0.12 | 0.45 | 0.30 | 0.12 | 0.52 | 0.11 | 0.01 | 0.27 |
| Unknown | 0.24 | 0.14 | 0.36 | 0.32 | 0.18 | 0.47 | 0.08 | 0.00 | 0.25 |
Adverse pregnancy events include preterm births and death/still birth or early terminated pregnancies; p: pooled proportion; CI: confidence interval; CRP: C-reactive protein; Analyses were performed only for outcomes where at least 2 studies were available; highlighted values indicate a significant presence (at least 6 %) of condition relative to their average value.
The percentages indicate high or low proportion of studies with a specific characteristics.
Systematic review of individual case analysis
Of the 45 infected women, the rate of C-section was 68.9 %. Of 43 newborns, 55.8 % (95 %CI: 39.9 %–70.9 %) had preterm birth, and 35 % (95 %CI: 20.6 %–51.7 %) had low birth weight (Fig. 3B). Lowest C-section rate with the highest preterm birth and low birth weight outcomes were observed in the US studies compared to Chinese, European, or studies from other countries (Fig. 3B). Among clinical signs and symptoms, fever (64.4 %) and cough (60 %) were the most common followed by lymphopenia (38.5 %), dyspnea (35.6 %), myalgia (24.4 %), sore throat (22.2 %), and fatigue (15.6 %). Among comorbidities, 8.1 % had preeclampsia and 21.1 % had gestational diabetes. Over half of the patients (55.3 %) had elevated CRP levels. In the treatment profile of reported studies, most of the patients received antiviral medications (68.6 %) and antibiotics (62.9 %). Oxygen support was given to 71.4 % of infected patients (Table 2). In newborns, pneumonia was in 6(16.7 %) and lymphopenia was in 4(12.5 %) and no neonates experienced asphyxia. Infection acquired at an early gestation week (≤ 35) was strongly associated with preterm birth (89.5 % vs. 22.7 %, p < 0.001) and low birth weight (62.5 % vs, 9.09 %, p = 0.0014). The presence of myalgia symptom was also associated with a high preterm birth rate (88.9 % vs. 43.8 %, p = 0.024). Only six (14 %) neonates had a COVID infection and 2 (5 %) were stillborn/death.
The heterogeneity in estimates was also observed according to the type of studies (case series vs. case reports). Women included in case report studies had higher proportions of symptoms particularly dyspnea, fatigue, sore throat, and myalgia compared to women included in case series studies. Moreover, infected patients in case report studies received more oxygen support (71 % vs. 31 %) and antivirals (69 % vs. 48 %) suggesting that case reports represent more severe infected pregnant cases (Table 2).
Comparative evaluations of current study with other studies
Table 5 displays the comparative evaluation of our study findings in relation to other existing systematic reviews. There were 12 other systematic reviews published on this topic [11,[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]]. All studies included a combined analysis of case series and case reports except one study [17]. Only one study reported qualitative data separately for case report and case series studies. The majority of prior studies performed descriptive data analysis except two studies. Of 12 studies, 5 studies only included Chinese studies [13,20,[14], [15], [16], [17], [18], [19], [20], [21], [22], [23]], 4 studies included data from up to four countries [11,14,16,17] while rest three studies included data from multiple countries [15,18,19] with predominantly case reports [15,18]. Compared to our study (n = 61), all studies were based on relatively a small number of studies (range: 8–49) with small sample sizes. Most studies reported higher rates and a wider range of C-section (median: 80 %, range: 55.6 %–94 % vs. 72 %), preterm birth (median: 34 %, range: 21.3 %–63.8 % vs. 23 %) and low birth weight (median: 9 %, range: 5.3 %–42.8 % vs. 7 %) and vertical transmission of infection (median 0 %, range: 0 %–11.5 % vs. 1 %) compared to current meta-analysis of case series studies.
Table 5.
Comparative evaluation of current systematic review and meta-analysis with other systematic reviews.
| Authors | Study design | Type of study(case series/case reports) | Number of studies | Sample size (mothers/neonates) | C-section | Preterm birth | LBW | Other events | Infection transmission | Included studies |
|---|---|---|---|---|---|---|---|---|---|---|
| Trocado [13] et al [77] | Systematic Review | Mixed (6/2) | 8 | 95/51 | 94 % | 35 % | 20 % | 1.9 % | 1.9 % | From China only |
| Thomas et al [14] | Systematic Scoping Review | Mixed (7/11) | 18 | 157/160 | 73 % | 20 % | 11 % | 1 % | 6 % | Limited countries |
| Juan et al [15] | Systematic Review | Mixed (9/15) | 24 | 324/221 | 78.1 % in case series | NR | 7.8 % in case reports | 1.4 % in case series; 0.5 % in case reports | 0.40 % | Multiple countries with predominantly case reports. Qualitative separate analysis |
| Matar et al [16] | Systematic Review | Mixed(14/10) | 24 | 136/94 | 76.30 % | 37.70 % | NR | NR | 11.50 % | Limited countries with weighted analysis without separate analysis for case reports and case series |
| Huntley et al [17] | Systematic Review | Case series (13) | 13 | 538/435 | 84.70 % | 20.10 % | NR | 0.30 % | 0 % | Limited countries without weighted analysis |
| Walker et al [18] | Systematic Review | Mixed (9/40) | 49 | 655/666 | 55.60 % | NR | NR | 1.20 % | 4 % | Multiple countries with predominantly case reports |
| Trippella et al [19] | Systematic Review | Mixed (18/19) | 37 | 275/248 | 75 % | 23 % | NR | 0.80 % | 8.40 % | Multiple countries |
| Smith et al [20] | Systematic Review | Mixed (4/5) | 9 | 92/60 | 80 % | 63.80 % | 42.80 % | 0.20 % | 0 % | From China only |
| Kasraeian et al [21] | Systematic review and meta-analysis | Mixed (NR) | 9 | 87/86 | NR | 61.20 % | NR | 0.40 % | 0.00 % | From China only with weighted analysis without separate analysis for case reports and case series |
| Yang et al [22] | Systematic Review | Mixed(10/8) | 18 | 114/84 | 91 % | 21.30 % | 5.30 % | 2.40 % | 0 % | From China only |
| Zaigham and Andersson [11] | Systematic Review | Mixed (4/14) | 18 | 108 | 91 % | NR | NR | 1.90 % | 0.10 % | Limited countries |
| Muhidin et al [23] | Systematic Review | Mixed (6/3) | 9 | 89 | 94 % | 33.70 % | 7.90 % | NR | 0 % | From China only |
| Current study | Meta-analysis | Case series (27) | 27 | 745/505 | 72 % (91 % in China, 40 % in US, 38 % in Europe) | 23 % (17 % in China, 12 % in US, 17 % in US) | 7% (5% in China) | 2.00 % | 1.00 % | Multiple countries, separate analysis by location, compared with controls, and association analysis with heterogeneity assessment |
| Systematic Review | Case reports (45) | 34 | 45/43 | 68.9 % (70 % in China, 50 % in US,78 % in Europe, and 75 % in others) | 56 % (45 % in China, 83 % in US,50 % in Europe, and 71 % in others) | 35 % (26 % in China, 67 % in US,44 % in Europe, and 17 % in others) | 5 % | 14 % | Multiple countries, separate analysis by location, and association analysis |
LBW: low birth weight; NR: not reported; Other events include deaths/still birth or early terminations.
Discussion
Our findings of this systematic review and meta-analysis confirmed that preterm birth and other adverse pregnancy outcomes are commonly observed in COVID-19 patients and relatively larger in Chinese and European studies compared to the US studies. The C-section rate was highest in Chinese studies compared to the US and European studies in both meta-analysis and systematic review. Moreover, the mode of delivery and adverse pregnancy outcomes were even higher in severe infected cases as reflected in the qualitative analysis of case reports and subgroup analyses of case series studies. The odds of preterm birth was even higher in COVID-19 patients than the controls as well as general populations [89]. The majority of the preterm birth or adverse pregnancy outcomes occurred if the infection was acquired early in the gestational ages (25–35 weeks). This was further confirmed by the case report studies in which the majority of adverse pregnancy outcomes occurred if the cases were detected early. This suggests that pregnant women should follow an intensive practice to avoid acquiring an infection during early gestational ages. However, if a pregnant female becomes infected by SARS-CoV-2, it needs to be detected as early as possible. Some symptoms particularly myalgia symptom at presentation was associated with adverse pregnancy outcomes in both analyses of case series and case report studies. Specific attentions are required for pregnant women presenting with myalgia symptom or requiring oxygen supports.
Case reports from Korea [55], India [72], Netherlands [82,83], Australia [88], Italy [84], Ireland [82], and Turkey [56] did not show any adverse pregnancy outcomes. All of these cases were detected after the gestation age of 35 weeks except for one case in a study [82]. However, cases from Peru [75], Spain [73], Iran [63,80], and UK [78] showed adverse pregnancy outcomes that may be due to early detection of infection before gestation age of 35 weeks. Out of eight cases from seven case reports reported from the US, six cases had proceeded to delivery [62,67,76,85]. Of which, two cases [62,85] diagnosed with an infection at a gestation age of more than 35 weeks and one observed with no adverse pregnancy outcomes and others observed with preterm birth. However, the other four cases [67,76,85] diagnosed before gestation age of 35 weeks and observed with preterm birth and low birth weight. These findings suggest that acquiring infection at an early gestational age might lead to adverse pregnancy outcomes. Although a case series study from the US detected infection as early as 32 gestational weeks [44], the reported preterm birth was only one out of 18 deliveries. Similarly, another US study [47] reported only two adverse events out of 8 deliveries even when all cases were identified prior to a gestational week of 35. However, it would be important to ascertain the pregnancy outcome status on the remaining 25 pregnant cases from Breslin et al. [44] and 34 pregnant cases from Lokken et al. [47] as well. One case study reporting two neonatal outcomes from Sweden [64] had also reported 1 neonate with preterm birth and low birth weight outcomes. It is known that the viral infection can induce adverse pregnancy outcomes including preterm birth and low birth weight [5]. These findings suggest that careful management is required to reduce the risk of adverse pregnancy outcomes among patients who acquired infection at an early gestational week.
In our meta-analysis, the adverse pregnancy event rate was estimated to be 27 % which was skewed by two studies. One study had 5 early pregnancy terminations due to severe complications such as shortness of breath, chest tightness, and psychosocial factors that led to pregnancy termination [38]. Another study had 3 threatened abortions due to COVID-19 [32]. Despite these observations, our case report analyses produced 56 % adverse pregnancy complications and 35 % low birth weight. Although these studies did not report the psychological health of the infected women, psychological health especially depression, anxiety, and stress of pregnant women have been associated with preterm birth including low birth weight [90,91]. Furthermore, an interplay between viral infections with poor psychological health may have deleterious effects on maternal and child health. It is important to evaluate the psychological health of COVID-infected pregnant women.
The C-section rate in infected patients was observed unusually higher than uninfected pregnant women. However, this could have been done to minimize severe adverse outcomes in mothers and neonates as suggested [92]. Similar to other studies [11], our study also identified cough, fever, and lymphopenia as the prevalent symptoms in infected patients. However, subgroup analyses in our study indicated that early detection of infection, especially during the asymptomatic phase, minimizes the risk of adverse outcomes. As observed in other studies [10,11], the majority of infected women received some antibiotic or antiviral therapies. However, studies reporting more patients with antiviral medications or oxygen support yielded a high proportion of adverse pregnancy outcomes. COVID infection acquiring at an early gestational age with some symptoms at presentation such as myalgia and lymphopenia for preterm birth while sore throat and diarrhea for low birth weight and a requirement of oxygen support may be used for risk assessments in pregnant women.
Our heterogeneity assessments revealed that the variation in the estimates was heavily influenced by the location, sample size, and severity of the studied COVID cases. Disproportionally higher rates of C-section and adverse pregnancy outcomes were observed in Chinese studies followed by European and US studies in the meta-analysis of case series studies. In contrast, the rates of preterm birth and low birth weight were higher in the US studies with the lowest C-section rate compared to case report studies from other countries. All patients reported in case reports from the US required oxygen support except one [70] compared to the studies from other locations indicating the reporting of more severe cases in the US studies. Nevertheless, a case report study mostly highlights the unusual and complex cases typically requiring critical management as evidence by the overall proportion of oxygen support given to patients and the prevalence of other symptoms at presentation in case reports compared to case series studies. Thus, our systematic review of case reports provides the estimates of adverse pregnancy outcomes by different locations in relatively severe COVID infected cases compared to estimates obtained from our meta-analysis. Since case reports are limited with one or a few cases, the proportion estimates from analysis of case reports may be biased. Compared to our study, only two studies [14,19] out of 12 studies yielded similar rates of C-section and preterm birth. However, these studies produced a fairly large rate of vertical transmission of infection compared to our study. All prior systematic reviews analyzed case series studies with case reports which is likely to produce biased estimates compared to our meta-analysis of case series studies.
Our study reports a 1 % prevalence of COVID-19 in neonates based on case series studies. Six neonates had COVID infection reported in case studies, one in vaginal delivery [63] and others in cesarean delivery [59,63,75,76,79,82]. The majority of mothers of these neonates had a fever, myalgia, other comorbidities, and received oxygen support and antibiotics. Of six, four neonates had a preterm birth and two neonates had an Apgar score of 0 and 6. Though it seems a rare transmission of infection in babies from mothers, more research is needed to examine the long-term outcomes of infection and delivery times and their interactions with maternal characteristics among infected mothers with neonates. Extra precaution is needed while breastfeeding until the infection is completely eliminated. In case reports, all the neonates had a 5-minutes Apgar score of 8 or higher except for three neonates, one died immediately after birth [63], and one within 2 h of birth [69]. Some neonates had pneumonia and lymphopenia which were resolved after treatment. The majority of the infants did not have serious morbidities requiring intensive care admission or ventilator supports.
There are several limitations to consider while interpreting findings from this systematic review and meta-analysis. Our study was based on mostly case series and case report studies with limited and heterogeneous sample sizes and approximately half of the included studies were carried out in China. These factors limit the generalizability of our findings. However, our sensitivity analysis for the primary outcomes based on location and sample size of the studies significantly reduced the heterogeneity in the estimates across the considered studies. Although our study included a fair number of case reports, the estimates obtained from case studies may be biased. Despite these limitations, our study is the first comprehensive study that provides the quantitative estimates of adverse pregnancy outcomes based on a relatively large sample size separately presented for case report and case series studies. Contrary to other review studies [10,11], our study included up-to-date data from the USA as well as other countries. The other systematic reviews are not up-to-high quality because of mixing case-reports with case-series articles without meta-analysis, and not determining heterogeneity in maternal and neonatal outcomes. Our study provided the heterogeneity assessment as well. We do not only report the prevalence of pregnancy outcomes among infected patients but also provide factors associated with adverse pregnancy outcomes separately for preterm, adverse pregnancy events, and low birth weight. Furthermore, our study also reports the prevalence of pregnancy outcomes by location (US, Europe, China, Others) and provides a comparison of preterm birth in relation to controls. Our subgroup analyses produced important clinical and treatment factors that could be considered for appropriate management of pregnant women with COVID-19 to achieve better outcomes.
Conclusions
Adverse pregnancy outcomes including low birth weight and cesarean-section were observed to be relatively higher in COVID-19 positive patients and relatively larger in Chinese and European studies compared to the US studies. The rates of pregnancy outcomes varied by location, type, and size of the studies. The rates of adverse pregancy outcomes were substantially higher in severe infected cases mostly reported in case report studies. Serious adverse events were uncommon in infected women and newborns. Vertical transmission of infection was uncommon in our study. Adverse pregnancy outcomes were associated with early detection of infection during pregnancy, more symptomatic presentation, myalgia symptom at presentation, and usage of supplemental oxygen therapy or antiviral medications. Regular screening for coronavirus infection, early identification of infection in asymptomatic pregnant women, and safe practice to avoid acquiring an infection during early gestational ages may be associated with more favorable pregnancy outcomes. Close monitoring and future surveillance studies are required for infected newborns and premature babies to evaluate their long-term adverse consequences of infection.
Funding
No financial support was received for this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We appreciate the efforts of all the researchers whose articles were included in this study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ejogrb.2020.07.034.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Hu Y., Sun J., Dai Z., Deng H., Li X., Huang Q., et al. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y., Mao B., Liang S., Yang J.W., Lu H.W., Chai Y.H., et al. Association between ages and clinical characteristics and outcomes of coronavirus disease 2019. Eur Respir J. 2020 [Google Scholar]
- 3.Liu H., Wang L.L., Zhao S.J., Kwak-Kim J., Mor G., Liao A.H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J Reprod Immunol. 2020;139 doi: 10.1016/j.jri.2020.103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12 doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Racicot K., Mor G. Risks associated with viral infections during pregnancy. J Clin Invest. 2017;127:1591–1599. doi: 10.1172/JCI87490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon A.K., Hollander G.A., McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282 doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsafaras G.P., Ntontsi P., Xanthou G. Advantages and limitations of the neonatal immune system. Front Pediatr. 2020;8:5. doi: 10.3389/fped.2020.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon J.Y., Romero R., Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387–390. doi: 10.1111/aji.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monteleone P.A., Nakano M., Lazar V., Gomes A.P., de H.M., Bonetti T.C. A review of initial data on pregnancy during the COVID-19 outbreak: implications for assisted reproductive treatments. JBRA Assist Reprod. 2020;24:219–225. doi: 10.5935/1518-0557.20200030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Della Gatta A.N., Rizzo R., Pilu G., Simonazzi G. COVID19 during pregnancy: a systematic review of reported cases. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020 doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panahi L., Amiri M., Pouy S. Risks of novel coronavirus disease (COVID-19) in pregnancy; a narrative review. Arch Acad Emerg Med. 2020;8:e34. [PMC free article] [PubMed] [Google Scholar]
- 13.Trocado V., Silvestre-Machado J., Azevedo L., Miranda A., Nogueira-Silva C. Pregnancy and COVID-19: a systematic review of maternal, obstetric and neonatal outcomes. J Matern Fetal Neonatal Med. 2020:1–13. doi: 10.1080/14767058.2020.1781809. [DOI] [PubMed] [Google Scholar]
- 14.Thomas P., Alexander P.E., Ahmed U., Elderhorst E., El-Khechen H., Mammen M.J., et al. Vertical transmission risk of SARS-CoV-2 infection in the third trimester: a systematic scoping review. J Matern Fetal Neonatal Med. 2020:1–8. doi: 10.1080/14767058.2020.1786055. [DOI] [PubMed] [Google Scholar]
- 15.Juan J., Gil M.M., Rong Z., Zhang Y., Yang H., Poon L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet Gynecol. 2020;56:15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matar R., Alrahmani L., Monzer N., Debiane L.G., Berbari E., Fares J., et al. Clinical presentation and outcomes of pregnant women with COVID-19: a systematic review and meta-analysis. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huntley B.J.F., Huntley E.S., Di Mascio D., Chen T., Berghella V., Chauhan S.P. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS-Co-V-2) infection: a systematic review. Obstet Gynecol. 2020 doi: 10.1097/AOG.0000000000004010. [DOI] [PubMed] [Google Scholar]
- 18.Walker K.F., O’Donoghue K., Grace N., Dorling J., Comeau J.L., Li W., et al. Maternal transmission of SARS-COV-2 to the neonate, and possible routes for such transmission: a systematic review and critical analysis. Bjog. 2020 doi: 10.1111/1471-0528.16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trippella G., Ciarcià M., Ferrari M., Buzzatti C., Maccora I., Azzari C., et al. COVID-19 in pregnant women and neonates: a systematic review of the literature with quality assessment of the studies. Pathogens. 2020;9 doi: 10.3390/pathogens9060485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith V., Seo D., Warty R., Payne O., Salih M., Chin K.L., et al. Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasraeian M., Zare M., Vafaei H., Asadi N., Faraji A., Bazrafshan K., et al. COVID-19 pneumonia and pregnancy; a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2020:1–8. doi: 10.1080/14767058.2020.1763952. [DOI] [PubMed] [Google Scholar]
- 22.Yang Z., Wang M., Zhu Z., Liu Y. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. J Matern Fetal Neonatal Med. 2020:1–4. doi: 10.1080/14767058.2020.1759541. [DOI] [PubMed] [Google Scholar]
- 23.Muhidin S., Behboodi Moghadam Z., Vizheh M. Analysis of maternal coronavirus infections and neonates born to mothers with 2019-nCoV; a systematic review. Arch Acad Emerg Med. 2020;8:e49. [PMC free article] [PubMed] [Google Scholar]
- 24.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 26.Cumpston M., Li T., Page M.J., Chandler J., Welch V.A., Higgins J.P., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10 doi: 10.1002/14651858.ED000142. ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dwivedi A.K., Shukla R. Evidence-based statistical analysis and methods in biomedical research (SAMBR) checklists according to design features. Cancer Rep. 2019:e1211. doi: 10.1002/cnr2.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D., Li L., Wu X., Zheng D., Wang J., Yang L., et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020:1–6. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 29.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen S., Liao E., Cao D., Gao Y., Sun G., Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020 doi: 10.1002/jmv.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X., et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X., Sun R., Chen J., Xie Y., Zhang S., Wang X. Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia. Int J Gynaecol Obstet. 2020 doi: 10.1002/ijgo.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020 doi: 10.1016/j.jinf.2020.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G., et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan S., Jun L., Nawsherwan Siddique R., Li Y., Han G., et al. Association of COVID-19 with pregnancy outcomes in health-care workers and general women. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W., Wang J., Li W., Zhou Z., Liu S., Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front Med. 2020 doi: 10.1007/s11684-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J., et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Lu J.Y., Min Wei, Ziheng Cheng, Xiaocui Zhou, Jun Li, Jinhua Tian, et al. Analysis of pregnancy outcomes of pregnant women during the epidemic of new coronavirus pneumonia in Hubei. Chin J Obst Gynecol. 2020;55 [Google Scholar]
- 39.Liu H., Liu F., Li J., Zhang T., Wang D., Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect. 2020;80:e7–e13. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K., et al. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liao J., He X., Gong Q., Yang L., Zhou C., Li J. Analysis of vaginal delivery outcomes among pregnant women in Wuhan, China during the COVID-19 pandemic. Int J Gynaecol Obstet. 2020 doi: 10.1002/ijgo.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C., Yang W., Wu X., Zhang T., Zhao Y., Ren W., et al. Clinical manifestation and laboratory characteristics of SARS-CoV-2 infection in pregnant women. Virol Sin. 2020:1–6. doi: 10.1007/s12250-020-00227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng Y., Lin L., Yan Q., Wei W., Xiang Yang B., Huang R., et al. Update on clinical outcomes of women with COVID-19 during pregnancy. Int J Gynaecol Obstet. 2020 doi: 10.1002/ijgo.13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breslin N., Baptiste C., Gyamfi-Bannerman C., Miller R., Martinez R., Bernstein K., et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blitz M.J., Rochelson B., Minkoff H., Meirowitz N., Prasannan L., London V., et al. Maternal mortality among women with coronavirus disease 2019 admitted to the intensive care unit. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.London V., McLaren R., Jr., Atallah F., Cepeda C., McCalla S., Fisher N., et al. The relationship between status at presentation and outcomes among pregnant women with COVID-19. Am J Perinatol. 2020 doi: 10.1055/s-0040-1712164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lokken E.M., Walker C.L., Delaney S., Kachikis A., Kretzer N.M., Erickson A., et al. Clinical characteristics of 46 pregnant women with a SARS-CoV-2 infection in Washington State. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sentilhes L., De Marcillac F., Jouffrieau C., Kuhn P., Thuet V., Hansmann Y., et al. COVID-19 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vivanti A.J., Mattern J., Vauloup-Fellous C., Jani J., Rigonnot L., El Hachem L., et al. Retrospective description of pregnant women infected with severe acute respiratory syndrome coronavirus 2. France. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2609.202144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrazzi E., Frigerio L., Savasi V., Vergani P., Prefumo F., Barresi S., et al. Vaginal delivery in SARS-CoV-2 infected pregnant women in Northern Italy: a retrospective analysis. Bjog. 2020 doi: 10.1111/1471-0528.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savasi V.M., Parisi F., Patanè L., Ferrazzi E., Frigerio L., Pellegrino A., et al. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2020 doi: 10.1097/AOG.0000000000003979. [DOI] [PubMed] [Google Scholar]
- 52.Govind A., Essien S., Karthikeyan A., Fakokunde A., Janga D., Yoong W., et al. Re: novel Coronavirus COVID-19 in late pregnancy: outcomes of first nine cases in an inner city London hospital. Eur J Obstet Gynecol Reprod Biol. 2020 doi: 10.1016/j.ejogrb.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pereira A., Cruz-Melguizo S., Adrien M., Fuentes L., Marin E., Perez-Medina T. Clinical course of coronavirus disease-2019 in pregnancy. Acta Obstet Gynecol Scand. 2020;99:839–847. doi: 10.1111/aogs.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., Seferovic M.D., Aski S.K., Arian S.E., et al. Maternal death due to COVID-19 disease. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee D.H., Lee J., Kim E., Woo K., Park H.Y., An J. Emergency cesarean section on severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) confirmed patient. Korean J Anesthesiol. 2020 doi: 10.4097/kja.20116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalafat E., Yaprak E., Cinar G., Varli B., Ozisik S., Uzun C., et al. Lung ultrasound and computed tomographic findings in pregnant woman with COVID-19. Ultrasound Obstet Gynecol. 2020 doi: 10.1002/uog.22034. [DOI] [PubMed] [Google Scholar]
- 57.Li Y., Zhao R., Zheng S., Chen X., Wang J., Sheng X., et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan C., Lei D., Fang C., Li C., Wang M., Liu Y., et al. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang S., Guo L., Chen L., Liu W., Cao Y., Zhang J., et al. A case report of neonatal COVID-19 infection in China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zambrano L.I., Fuentes-Barahona I.C., Bejarano-Torres D.A., Bustillo C., Gonzales G., Vallecillo-Chinchilla G., et al. A pregnant woman with COVID-19 in Central America. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 Novel Coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iqbal Sn, Overcash R., Mokhtari N., Saeed H., Gold S., Auguste T., et al. An uncomplicated delivery in a patient with Covid-19 in the United States. N Engl J Med. 2020;382:e34. doi: 10.1056/NEJMc2007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karami P., Naghavi M., Feyzi A., Aghamohammadi M., Novin Ms, Mobaien A., et al. Mortality of a pregnant patient diagnosed with COVID-19: a case report with clinical, radiological, and histopathological findings. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gidlof S., Savchenko J., Brune T., Josefsson H. COVID-19 in pregnancy with comorbidities: more liberal testing strategy is needed. Acta Obstet Gynecol Scand. 2020 doi: 10.1111/aogs.13862. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y., Peng H., Wang L., Zhao Y., Zeng L., Gao H., et al. Infants born to mothers with a new coronavirus (COVID-19) Front Pediatr. 2020;8:104. doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen S., Huang B., Luo D.J., Li X., Yang F., Zhao Y., et al. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi. 2020;49:E005. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 67.Blauvelt Ca, Chiu C., Donovan Al, Prahl M., Shimotake Tk, George Rb, et al. Acute respiratory distress syndrome in a preterm pregnant patient with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2020 doi: 10.1097/AOG.0000000000003949. [DOI] [PubMed] [Google Scholar]
- 68.Hong L., Smith N., Keerthy M., Lee-Griffith M., Garcia R., Shaman M., et al. Severe COVID-19 infection in pregnancy requiring intubation without preterm delivery: a case report. Case Rep Womens Health. 2020 doi: 10.1016/j.crwh.2020.e00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J., Wang Y., Zeng Y., Song T., Pan X., Jia M., et al. Critically ill pregnant patient with COVID-19 and neonatal death within two hours of birth. Int J Gynaecol Obstet. 2020 doi: 10.1002/ijgo.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Browne P.C., Linfert J.B., Perez-Jorge E. Successful treatment of preterm labor in association with acute COVID-19 infection. Am J Perinatol. 2020 doi: 10.1055/s-0040-1709993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu D., Sang L., Du S., Li T., Chang Y., Yang X.A. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. J Med Virol. 2020 doi: 10.1002/jmv.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma Ka, Kumari R., Kachhawa G., Chhabra A., Agarwal R., Sharma Ka, et al. Management of the first patient with confirmed COVID-19 in pregnancy in India: from guidelines to frontlines. Int J Gynaecol Obstet. 2020 doi: 10.1002/ijgo.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.González Romero D., Ocampo Pérez J., González Bautista L., Santana-Cabrera L. Pregnancy and perinatal outcome of a woman with COVID-19 infection. Rev Clin Esp. 2020 doi: 10.1016/j.rce.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peng Z., Wang J., Mo Y., Duan W., Xiang G., Yi M., et al. Unlikely SARS-CoV-2 vertical transmission from mother to child: a case report. J Infect Public Health. 2020;13:818–820. doi: 10.1016/j.jiph.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Valdez L.M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020 doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mehta H., Ivanovic S., Cronin A., VanBrunt L., Mistry N., Miller R., et al. Novel coronavirus-related acute respiratory distress syndrome in a patient with twin pregnancy: a case report. Case Rep Womens Health. 2020;27:e00220. doi: 10.1016/j.crwh.2020.e00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu Y., Fan C., Bian J. YinShen. Severe COVID-19 in a pregnant patient admitted to hospital in Wuhan. Int J Gynaecol Obstet. 2020 doi: 10.1002/ijgo.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cooke W.R., Billett A., Gleeson S., Jacques A., Place K., Siddall J., et al. SARS-CoV-2 infection in very preterm pregnancy: experiences from two cases. Eur J Obstet Gynecol Reprod Biol. 2020;250:259–260. doi: 10.1016/j.ejogrb.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kirtsman M., Diambomba Y., Poutanen S.M., Malinowski A.K., Vlachodimitropoulou E., Parks W.T., et al. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ. 2020;192:E647–e50. doi: 10.1503/cmaj.200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taghizadieh A., Mikaeili H., Ahmadi M., Valizadeh H. Acute kidney injury in pregnant women following SARS-CoV-2 infection: a case report from Iran. Respir Med Case Rep. 2020;30 doi: 10.1016/j.rmcr.2020.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khan S., Peng L., Siddique R., Nabi G., Nawsherwan Xue M., et al. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect Control Hosp Epidemiol. 2020;41:748–750. doi: 10.1017/ice.2020.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fontanella F., Hannes S., Keating N., Martyn F., Browne I., Briet J., et al. COVID-19 infection during the third trimester of pregnancy: current clinical dilemmas. Eur J Obstet Gynecol Reprod Biol. 2020;251:268–271. doi: 10.1016/j.ejogrb.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grimminck K., Santegoets L.A.M., Siemens F.C., Fraaij P.L.A., Reiss I.K.M., Schoenmakers S. No evidence of vertical transmission of SARS-CoV-2 after induction of labour in an immune-suppressed SARS-CoV-2-positive patient. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-235581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferraiolo A., Barra F., Kratochwila C., Paudice M., Vellone V.G., Godano E., et al. Report of positive placental swabs for SARS-CoV-2 in an asymptomatic pregnant woman with COVID-19. Medicina (Kaunas) 2020;56 doi: 10.3390/medicina56060306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Silverstein J.S., Limaye M.A., Brubaker S.G., Roman A.S., Bautista J., Chervenak J., et al. Acute respiratory decompensation requiring intubation in pregnant women with SARS-CoV-2 (COVID-19) AJP Rep. 2020;10:e169–e175. doi: 10.1055/s-0040-1712925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.AlZaghal L.A., AlZaghal N., Alomari S.O., Obeidat N., Obeidat B., Hayajneh W.A. Multidisciplinary team management and cesarean delivery for a Jordanian woman infected with SARS-COV-2: a case report. Case Rep Womens Health. 2020;27 doi: 10.1016/j.crwh.2020.e00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anderson J., Schauer J., Bryant S., Graves Cr. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: a case report. Case Rep Womens Health. 2020;27:e00221. doi: 10.1016/j.crwh.2020.e00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lowe B., Bopp B. COVID-19 vaginal delivery - A case report. Aust N Z J Obstet Gynaecol. 2020;60:465–466. doi: 10.1111/ajo.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.World Health Organization . WHO; Geneva, Switzerland: 2015. WHO recommendations on interventions to improve preterm birth outcomes. 2015. [PubMed] [Google Scholar]
- 90.Staneva A., Bogossian F., Pritchard M., Wittkowski A. The effects of maternal depression, anxiety, and perceived stress during pregnancy on preterm birth: a systematic review. Women Birth. 2015;28:179–193. doi: 10.1016/j.wombi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Ruiz R.J., Dwivedi A.K., Mallawaarachichi I., Balcazar H.G., Stowe R.P., Ayers K.S., et al. Psychological, cultural and neuroendocrine profiles of risk for preterm birth. BMC Pregnancy Childbirth. 2015;15:204. doi: 10.1186/s12884-015-0640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma D., Spearman P. The impact of cesarean delivery on transmission of infectious agents to the neonate. Clin Perinatol. 2008;35:407–420. doi: 10.1016/j.clp.2008.03.010. vii-viii. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.