Abstract
Aims
Nutritional status as well as physical capacity is related to prognosis in patients with heart failure. The purpose of this study was to explore a simple prognostic indicator in patients with acute decompensated heart failure (ADHF) by including both nutritional status and physical capacity.
Methods and results
Patients hospitalized with ADHF (N = 203; mean age, 81 years) were enrolled. We evaluated the geriatric nutritional risk index (GNRI) on hospital admission and at discharge. A GNRI score < 92 was defined as malnutrition. Physical capacity was evaluated by simple walking test to determine if patients could walk 200 m, with a Borg scale score ≤ 13, without critical changes in vital signs. Primary endpoints were mortality and heart failure rehospitalization within 2 years. A total of 49% and 48% of patients showed malnutrition on admission and at discharge, respectively. Malnutrition at discharge was more strongly related to mortality [hazard ratio (HR) 3.382, 95% confidence interval (CI) 1.900–6.020, P < 0.0001)] than that on admission (HR 2.448, 95% CI 1.442–4.157, P = 0.001) by univariable analysis. Malnutrition at discharge was related to mortality (HR 2.370, 95% CI 1.166–4.814, P = 0.02), but malnutrition on admission was not related (HR 1.538, 95% CI 0.823–2.875, P = 0.18) by multivariable analysis. Almost half of patients (45%) could not walk 200 m, which was significantly related to mortality by univariable analysis (HR 3.303, 95% CI 1.905–5.727, P < 0.0001), but was not by multivariable analysis (HR 1.990, 95% CI 0.999–3.962, P = 0.05). The combined index including both GNRI and simple walking test was an independent and stronger predictor of mortality than either index alone by multivariable analysis (HR 2.249, 95% CI 1.362–3.716, P < 0.01). Neither malnutrition nor low physical capacity was related to heart failure rehospitalization by univariable analysis (HR 0.702, 95% CI 0.483–1.020, P = 0.06; HR 1.047, 95% CI 0.724–1.515, P = 0.81, respectively). Malnutrition at discharge significantly reduced heart failure rehospitalization by multivariable analysis (HR 0.431, 95% CI 0.266–0.698, P < 0.01). When patients were classified into Group G (both nutritional status and physical capacity at discharge were good), Group E (either was good), and Group B (both were bad), mortality rates were significantly different among the groups (log rank P < 0.0001).
Conclusion
A simple indicator including both nutritional status and physical capacity may predict 2 year mortality in elderly patients with ADHF.
Keywords: Geriatric nutritional risk index, Simple walking test, Acute decompensated heart failure, Elderly patients
Introduction
Malnutrition and reduced physical capacity are common in elderly patients with heart failure, and both have been associated with adverse outcomes. 1 , 2 Malnutrition exists in 20–42% of patients with heart failure. 2 , 3 Heart failure causes a low nutritional status due to malabsorption by intestinal oedema and hyperpermeability, along with anorexia, increased energy consumption, and anabolism. According to the cycle of frailty, malnutrition leads to sarcopenia. 4 At the same time, reduced physical capacity occurs as a result of sarcopenia and frailty. 4 , 5 , 6 However, the prognostic impact of an index incorporating both nutritional status and physical capacity in elderly patients with acute decompensated heart failure (ADHF) remains unknown. The purpose of this study was to explore a clinically feasible prognostic indicator in these patients by incorporating both nutritional status and physical capacity.
Methods
Subjects and study protocol
This retrospective and observational study enrolled 203 consecutive patients with ADHF at the National Hospital Organization Osaka National Hospital between April 2015 and January 2017. The investigation conformed to the principles outlined in the Declaration of Helsinki, and the National Hospital Organization Osaka National Hospital Institutional Review Board #2 approved the protocol (Approval No. 18026).
Acute decompensated heart failure was defined as the rapid onset or worsening of clinical symptoms/signs of congestion and/or peripheral hypoperfusion according to European Society of Cardiology guidelines. 7 We excluded 26 patients who did not undergo cardiac rehabilitation because their condition was serious at the time of admission; all 26 died during hospitalization. We also excluded patients in whom we could not calculate the geriatric nutritional risk index (GNRI) or if medical records had insufficient data.
We evaluated GNRI at hospital admission and at discharge. A GNRI score < 92 was defined as a high risk of malnutrition. 8 We performed simple walking test (SWT) at discharge. SWT <200 m was defined as reduced physical capacity. We also classified patients into three groups: Group G (both nutritional status and physical capacity at discharge were good), Group E (either variable was good), and Group B (both variables were bad) (Figure 1 ). Patients were followed up until 1 September 2019. Survival data were obtained by physicians at the hospital or in the outpatient setting. The clinical endpoints were all‐cause mortality and heart failure rehospitalization within 2 years of discharge.
Figure 1.
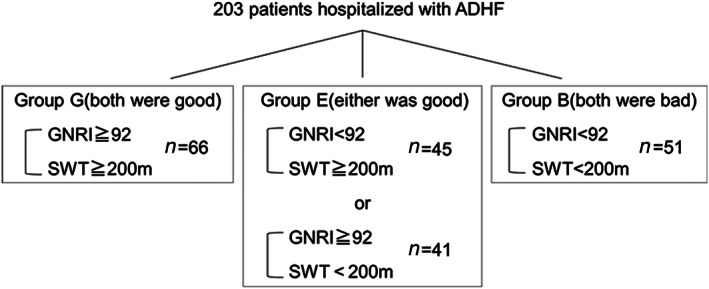
Study population. We divided patients into the three groups: Group G (both nutritional status and physical capacity at discharge were good), Group E (either variable was good), and Group B (both variables were bad).
We classified baseline heart diseases into dilated cardiomyopathy, hypertrophic cardiomyopathy, hypertensive heart disease, ischemic cardiomyopathy, valvular diseases, and other (sarcoidosis, amyloidosis, and cardiomyopathy based on arrhythmia). Each diagnosis was made by a cardiologist. Patients with more than moderate regurgitation and stenosis in the mitral or the aortic valves were classified as having valvular heart disease. Comorbidities were assessed based on medical records. Standard laboratory tests were assessed on hospital admission and at discharge. Echocardiography was performed at discharge. Left ventricular ejection fraction (EF) was evaluated by the modified Simpson method for patients with segmental asynergy and by the Teichholz method for patients without segmental asynergy. 9 We also checked the number of previous hospitalizations for heart failure from medical records.
Nutritional assessment
Nutritional status was assessed by the GNRI. GNRI was calculated as 14.89 × serum albumin (g/dL) + 41.7 × [present body weight (kg)/height (m)2]/22 = 14.89 × serum albumin (g/dL) + 41.7 × body mass index (BMI)/22. A GNRI score ≥ 98 was considered normal, a score ≥92 to <98 represented a low risk of malnutrition, ≥82 to <92 represented a moderate risk, and <82 represented a severe risk. 8 We defined patients with a GNRI score < 92 as the high‐risk nutritional group and those with a GNRI score ≥ 92 as the low‐risk nutritional group according to earlier reports. 10 , 11
Simple walking test
We evaluated physical capacity and ambulatory status according to SWT during cardiac rehabilitation. 12 Patients hospitalized due to ADHF expanded their levels of activity using the following protocol: patients started with a stepping exercise. If a patient could step more than 20 times without critical changes in vital signs and with a Borg scale ≤13 for leg fatigue and shortness of breath, they were considered to have passed the activity level. The Borg scale is a simple method to evaluate subjective symptoms. Cardiac rehabilitation exercises in which patients perform exercise with a low to moderate intensity and achieve a Borg scale score of 11–13. 13 , 14 The next level of activity was walking in the patient room, and each time the patient passed the SWT, we gradually extended the walking distance to 50, 100, and 200 m. When they would walk 200 m without resting or critical changes in vital signs and with a Borg scale ≤13 regardless of speed, patients proceeded to bicycle ergometer exercise.
Statistical analysis
Continuous data are expressed as mean ± standard deviation (SD). Independent samples t‐tests were used for comparison of continuous variables, and χ 2 tests were used for categorical variables. Cox hazard univariable and multivariable analyses were performed to assess the predictive value of GNRI and SWT for all‐cause mortality and heart failure rehospitalization. For Cox hazard analysis of the combined index including both GNRI and SWT, analysis was performed with Groups B, E, and G as continuous variables 0, 1, and 2. The multivariable analysis model included well‐established major confounders, such as age, brain natriuretic peptide (BNP), haemoglobin, and C‐reactive protein. Event‐free survival after discharge was estimated using the Kaplan–Meier analysis and compared using the log‐rank test. A P value <0.05 was considered statistically significant. All statistical analyses were performed using MedCalc (version 18.2.1, Ostend, Belgium).
Results
A total of 203 patients were enrolled. Median follow‐up period was 948 days [interquartile range (IQR): 493–1195]. Twenty patients dropped out during follow‐up within 2 years from discharge.
Mean GNRI on admission was 99 ± 12, and 49% patients had a GNRI score < 92. Mean BMI and albumin on admission were higher than that at discharge (mean BMI; 23.4 ± 5.1 vs. 21.5 ± 4.8 kg/m2, P < 0.0001, mean albumin; 3.7 ± 0.5 vs. 3.5 ± 0.5 g/l, P < 0.0001). On admission, mean BNP was 813 ± 941 pg/mL, mean haemoglobin was 11.3 ± 2.0 g/dL, mean estimated glomerular filtration rate (eGFR) was 41 ± 19 mL/min/1.73m2, and mean C‐reactive protein was 1.01 ± 5.06 mg/dL.
Table 1 shows the clinical characteristics of the patients. Patient mean age was 81 years, and there were 126 men (62%). EF was 50 ± 17%, and mean BNP was 276 pg/mL at discharge. A total of 85% of patients had chronic kidney disease (eGFR <60 mL/min/1.73m2) with an mean eGFR of 39 mL/min/1.73m2. Mean GNRI score at discharge was 93 ± 12, and 48% of patients had a GNRI score < 92. In the group with a GNRI score < 92 at discharge, the mean age was older, mean BNP and C‐reactive protein were higher, and mean haemoglobin was lower than the group with GNRI score ≥ 92 (P < 0.01). At discharge, 92 patients (45%) could not walk 200 m. In the group with SWT <200 m, the mean age was older (P < 0.01), mean BMI and haemoglobin were lower (P = 0.01, P < 0.01, respectively), mean EF and C‐reactive protein were higher (P = 0.03, P < 0.01, respectively), and more patients had chronic obstructive pulmonary disease when compared with the group with SWT ≥200 m (P = 0.01). Additionally, the group with SWT <200 m had a higher incidence of past hospitalizations for heart failure than the group with SWT ≥200 m (P < 0.01).
Table 1.
Patient characteristics
| Variables | Total cohort | GNRI <92 | GNRI ≥92 | P value | SWT <200 m | SWT ≥200 m | P value |
|---|---|---|---|---|---|---|---|
| (n = 203) | (n = 97) | (n = 106) | (n = 92) | (n = 111) | |||
| Age (years) | 81 ± 9 | 84 ± 7 | 79 ± 9 | <0.01 | 84 ± 10 | 78 ± 9 | <0.01 |
| Men (%) | 62 | 52 | 68 | 0.02 | 52 | 68 | 0.03 |
| BMI (kg/m2) | 21.5 ± 4.8 | 18.5 ± 2.5 | 24.2 ± 4.7 | <0.01 | 20.5 ± 4.8 | 22.3 ± 4.6 | 0.01 |
| Comorbidities | |||||||
| Atrial fibrillation (%) | 45 | 47 | 42 | 0.49 | 47 | 42 | 0.53 |
| Hypertension (%) | 68 | 59 | 75 | 0.02 | 64 | 70 | 0.35 |
| Diabetes mellitus (%) | 33 | 30 | 36 | 0.35 | 28 | 38 | 0.15 |
| COPD (%) | 9 | 7 | 8 | 0.75 | 13 | 4 | 0.01 |
| DCM (%) | 8 | 7 | 7 | 0.83 | 2 | 11 | 0.02 |
| HCM (%) | 5 | 3 | 7 | 0.17 | 5 | 5 | 0.99 |
| HHD (%) | 37 | 35 | 37 | 0.77 | 30 | 41 | 0.11 |
| ICM (%) | 23 | 19 | 25 | 0.27 | 24 | 21 | 0.59 |
| Valvular disease (%) | 32 | 40 | 23 | 0.01 | 33 | 30 | 0.66 |
| Other (%) | 21 | 21 | 22 | 0.78 | 20 | 22 | 0.71 |
| Medication | |||||||
| ACEi/ARB (%) | 59 | 55 | 61 | 0.45 | 49 | 66 | 0.02 |
| Beta blockers (%) | 66 | 67 | 63 | 0.55 | 55 | 72 | 0.01 |
| Diuretics (%) | 94 | 93 | 93 | 0.96 | 95 | 91 | 0.33 |
| Pimobendan (%) | 25 | 29 | 20 | 0.11 | 30 | 19 | 0.06 |
| Laboratory variables | |||||||
| BNP (pg/mL) | 276 ± 334 | 354 ± 434 | 207 ± 186 | <0.01 | 324 ± 416 | 237 ± 242 | 0.07 |
| Haemoglobin (g/dL) | 11.5 ± 1.9 | 10.7 ± 1.7 | 12.1 ± 1.9 | <0.01 | 11.0 ± 1.9 | 11.9 ± 1.8 | <0.01 |
| Urea (mg/dL) | 32 ± 16 | 32 ± 15 | 32 ± 17 | 0.83 | 33 ± 15 | 31 ± 17 | 0.31 |
| eGFR (mL/min/1.73m2) | 39 ± 17 | 41 ± 18 | 38 ± 17 | 0.15 | 38 ± 18 | 39 ± 19 | 0.79 |
| Sodium (mEq/L) | 139 ± 6 | 138 ± 8 | 139 ± 5 | 0.50 | 139 ± 4 | 138 ± 8 | 0.27 |
| C‐reactive protein (mg/dL) | 0.44 ± 0.71 | 0.59 ± 0.91 | 0.32 ± 0.44 | <0.01 | 0.59 ± 0.89 | 0.31 ± 0.49 | <0.01 |
| Albumin (g/L) | 3.5 ± 0.5 | 3.3 ± 0.40 | 3.8 ± 0.36 | <0.01 | 3.4 ± 0.5 | 3.7 ± 0.4 | <0.01 |
| Lymphocytes (mm3) | 1533 ± 557 | 1373 ± 513 | 1677 ± 558 | <0.01 | 1418 ± 514 | 1628 ± 575 | <0.01 |
| Echocardiography indices | |||||||
| EF (%) | 50 ± 17 | 49 ± 17 | 52 ± 18 | 0.25 | 54 ± 17 | 48 ± 17 | 0.03 |
| LVDd (mm) | 52 ± 9 | 51 ± 9 | 53 ± 9 | 0.32 | 51 ± 10 | 53 ± 8 | 0.09 |
| LVMI (g/m2) | 135 ± 49 | 135 ± 45 | 135 ± 52 | 0.97 | 142 ± 60 | 130 ± 39 | 0.17 |
| RWT | 0.38 ± 0.10 | 0.37 ± 0.10 | 0.39 ± 0.10 | 0.20 | 0.39 ± 0.13 | 0.37 ± 0.07 | 0.37 |
| E/A | 0.96 ± 0.70 | 0.88 ± 0.63 | 1.02 ± 0.77 | 0.39 | 0.81 ± 0.37 | 1.05 ± 0.84 | 0.12 |
| E/E' | 17 ± 8 | 17 ± 8 | 17 ± 7 | 0.81 | 19 ± 8 | 16 ± 7 | 0.08 |
| TrPG (mmHg) | 30 ± 12 | 29 ± 10 | 31 ± 14 | 0.31 | 32 ± 11 | 29 ± 13 | 0.33 |
| Prior HF hospitalizations (≥3 times) (%) | 37 | 41 | 34 | 0.30 | 49 | 27 | <0.01 |
ACEi, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; DCM, dilated cardiomyopathy; EF, ejection fraction; eGFR, estimate glomerular filtration rate; GNRI, geriatric nutritional risk index; HCM, hypertrophic cardiomyopathy; HF, heart failure; HHD, hypertensive heart failure; ICM, ischemic cardiomyopathy; LVDd, left ventricular end diastolic diameter; LVMI, left ventricular mass index; RWT, relative wall thickness; SWT, simple walking test.
Table 2 shows data on patients on passing the activity level on the SWT. There were 10 patients with a severely low physical capacity, including four patients who were unable to walk. A total of 111 patients passed the 200 m walk. Mean time between admission and starting rehabilitation was 4 ± 3 days; rehabilitation was continued until discharge. Mean total length of hospitalization was 24 ± 14 days.
Table 2.
Passing the activity level on the simple walking test
| n | Age (years) | Men (%) | GNRI | EF (%) | Hb (g/dL) | |
|---|---|---|---|---|---|---|
| Walking within room | 10 | 88 ± 4 | 20 | 76 ± 9 | 47 ± 17 | 10.9 ± 3.2 |
| Walking within 50 m | 17 | 86 ± 7 | 53 | 87 ± 10 | 55 ± 15 | 10.9 ± 1.7 |
| Walking within 100 m | 23 | 85 ± 7 | 52 | 94 ± 13 | 54 ± 22 | 10.7 ± 1.4 |
| Walking within 200 m | 42 | 84 ± 7 | 60 | 92 ± 12 | 54 ± 15 | 11.2 ± 1.9 |
| Walking over 200 m | 111 | 78 ± 9 | 68 | 97 ± 11 | 48 ± 17 | 11.9 ± 1.8 |
GNRI, geriatric nutritional risk index; EF, ejection fraction; Hb, haemoglobin..
All‐cause death occurred in 58 patients within 2 years of discharge, including cardiovascular death (n = 17; 14 heart failure, 2 cerebral haemorrhage, 1 aortic dissection) and non‐cardiovascular death (n = 41; 19 infection, 6 senility, 2 haemorrhagic shock, 14 unknown). Heart failure rehospitalization occurred in 115 patients.
Table 3 shows findings on the univariable and multivariable Cox hazard analysis for mortality adjusted for the combined index, age, BNP, haemoglobin, and C‐reactive protein. Only the combined index was an independent predictor of all‐cause mortality [hazard ratio (HR) 2.249, 95% confidence interval (CI) 1.362–3.716, P < 0.01)].
Table 3.
Univariable and multivariable Cox hazard model for mortality
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years) | 1.071 (1.033–1.109) | <0.01 | 1.024 (0.979–1.072) | 0.31 |
| BNP (pg/mL) | 1.001 (1.000–1.001) | <0.01 | 1.001 (0.999–1.001) | 0.13 |
| Haemoglobin (g/dL) | 0.803 (0.695–0.928) | <0.01 | 0.954 (0.811–1.123) | 0.57 |
| C‐reactive protein (mg/dL) | 1.310 (0.980–1.751) | 0.07 | 1.144 (0.839–1.561) | 0.39 |
| combined index (GNRI + SWT) | 3.005 (2.051–4.404) | <0.0001 | 2.249 (1.362–3.716) | <0.01 |
BNP, brain natriuretic peptide; CI, confidence interval; GNRI, geriatric nutritional risk index; HR, hazard ratio; SWT, simple walking test.
Table 4 shows Cox hazard univariable and multivariable analysis for mortality and heart failure rehospitalization adjusted for age, BNP, haemoglobin, and C‐reactive protein. A GNRI score < 92 at discharge was more strongly related to mortality (HR 3.382, 95% CI 1.900–6.020, P < 0.0001) than that on admission (HR 2.448, 95% CI 1.442–4.157, P = 0.001) by univariable analysis. GNRI score < 92 at discharge was related to mortality (HR 2.370, 95% CI 1.166–4.814, P = 0.02), but the score on admission was not related (HR 1.538, 95% CI 0.823–2.875, P = 0.18) by multivariable analysis. SWT <200 m and the combined index including both GNRI and SWT were also significantly related to mortality by univariable analysis (HR 3.303, 95% CI 1.905–5.727, P < 0.0001; HR 3.005, 95% CI 2.051–4.404, P < 0.0001, respectively). The combined index was a stronger predictor of the prognosis than either index alone by multivariable analysis (HR 2.249, 95% CI 1.362–3.716, P < 0.01). On the other hand, neither GNRI score < 92 nor SWT <200 m at discharge was related to heart failure rehospitalization by univariable analysis (HR 0.702, 95% CI 0.483–1.020, P = 0.06; HR 1.047, 95% CI 0.724–1.515, P = 0.81, respectively). Furthermore, GNRI score < 92 at discharge reduced heart failure rehospitalization by multivariable analysis (HR 0.431, 95% CI 0.266–0.698, P < 0.01).
Table 4.
Cox hazard model for mortality and heart failure rehospitalization
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Mortality (n = 58) | ||||
| GNRI < 92 at admission | 2.448 (1.442–4.157) | 0.001 | 1.538 (0.823–2.875) | 0.18 |
| GNRI < 92 at discharge | 3.382 (1.900–6.020) | <0.0001 | 2.370 (1.166–4.814) | 0.02 |
| SWT < 200 m | 3.303 (1.905–5.727) | <0.0001 | 1.990 (0.999–3.962) | 0.05 |
| Combined index (GNRI + SWT) | 3.005 (2.051–4.404) | <0.0001 | 2.249 (1.362–3.716) | <0.01 |
| Rehospitalization (n = 115) | ||||
| GNRI < 92 at admission | 0.776 (0.518–1.161) | 0.22 | 0.585 (0.362–0.946) | 0.03 |
| GNRI < 92 at discharge | 0.702 (0.483–1.020) | 0.06 | 0.431 (0.266–0.698) | <0.01 |
| SWT < 200 m | 1.047 (0.724–1.515) | 0.81 | 0.950 (0.617–1.464) | 0.82 |
| Combined index (GNRI + SWT) | 0.859 (0.660–1.118) | 0.26 | 0.673 (0.488–0.927) | 0.02 |
The multivariable analysis model included age, brain natriuretic peptide, haemoglobin and C‐reactive protein.
BNP, brain natriuretic peptide; CI, confidence interval; GNRI, geriatric nutritional risk index; HR, hazard ratio; SWT, simple walking test.
Figure 2 shows the Kaplan–Meier survival analysis for all‐cause mortality among the three groups. The 2 year mortality rate of Group G (both nutritional status and physical capacity at discharge were good) was 9%, that of Group E (either variable was good) was 27%, and that of Group B (both variables were bad) was 57%. Mortality rates were significantly different among the groups (log rank P < 0.0001).
Figure 2.
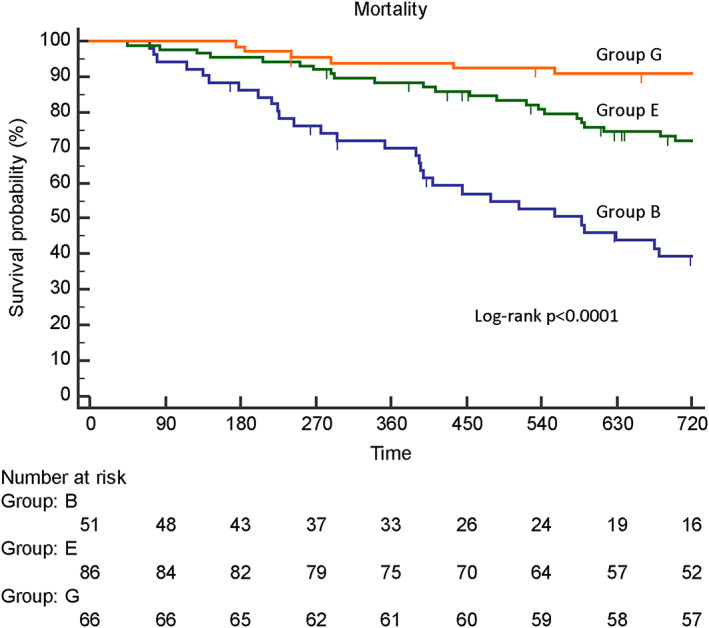
Kaplan–Meier curves for survival probability among the three groups. Group G, both nutritional status and physical capacity at discharge were good; Group E, either variable was good; Group B, both variables were bad.
Discussion
The results from this study indicate that (i) malnutrition and reduced physical capacity were frequently observed in elderly patients with ADHF; (ii) malnutrition and reduced physical capacity were predictors of all‐cause mortality; (iii) the combined index incorporating both nutritional status and physical capacity was an independent predictor of prognosis in elderly patients with ADHF, and it was a stronger predictor than either index alone.
Nutritional status
In our study, 48% of patients had malnutrition (GNRI <92) at discharge. This ratio was similar to that reported in an earlier study. 15 The Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition and the European Society of Clinical Nutritional and Metabolism recommend that a diagnosis of malnutrition should not be made based on biochemical data, because inflammation is considered the major reason for reduced serum albumin. 16 , 17 They reported that the relevance of laboratory tests of acute phase protein levels as indicators of malnutrition is limited. 16 , 17 In this study, GNRI was calculated using albumin; thus, a low GNRI score may not represent only malnutrition. In fact, C‐reactive protein in group with GNRI score < 92 was higher than that of GNRI score ≥ 92. Malnutrition in heart failure has been related to cardiac cachexia. Mechanisms of cardiac cachexia are multifactorial, involving neurohormonal disorders, overexpression of proinflammatory cytokines, increased oxidative stress, and an imbalance between anabolic and catabolic states. Diagnostic criteria of cardiac cachexia from the Cachexia Consensus Working Group include elevated C‐reactive protein and decreased haemoglobin. 18 In our study, inflammation and anaemia were found in the group with a GNRI score < 92.
Malnutrition was related to mortality in this study, which is similar to findings in another study. 19 One study used data on admission for the measurement of GNRI, 10 and another study used data at discharge for GNRI. 1 Thus, in terms of nutritional status on admission and at discharge in these studies, it was unclear which value was more relevant in the prediction of mortality. We assessed GNRI on admission and at discharge in this study and showed that malnutrition at discharge was more strongly related to mortality than that on admission.
In one study, malnutrition was related not only to the mortality but also to heart failure rehospitalization. 3 However, in our study, malnutrition reduced heart failure rehospitalization by multivariable analysis. Several factors could explain these findings. For example, it is possible that patients without malnutrition in this study did not comply with salt and water restrictions, which are a common cause of heart failure rehospitalization in patients with chronic heart failure. Alternatively, many patients with malnutrition died from non‐cardiovascular events. In addition, patient characteristics may also be different from the earlier studies. In fact, many old patients with advanced renal dysfunction were included in this study.
Physical capacity
Almost half of patients (45%) could not walk 200 m mostly because of sarcopenia and sometimes because of tachycardia and hypoxia. In patients with chronic obstructive pulmonary disease, dyspnoea and hypoxia were the main causes of inability to walk 200 m. Patients with heart failure commonly report reduced physical capacity, and reduced physical capacity in patients with heart failure has been associated with worse prognosis. 7 Peak oxygen uptake on the cardiopulmonary exercise test (CPX) is the gold standard for the assessment of exercise capacity. 20 However, the CPX is not always applicable to patients with ADHF, especially during hospitalization. The walking test is useful to evaluate physical capacity in such conditions, because there is no need for special equipment, and it is applicable to all patients even if they cannot perform the ergometer exercise because of extremely decreased exercise capacity. The 6‐min walking test (6MWT) is a widely used measure of physical capacity, and it was reported that the 6MWT provides prognostic utility comparable with the CPX. 21 Curtis et al. 22 showed that a 6MWT score < 200 m could identify patients with heart failure who are at markedly increased risk of death. In our study, we performed SWT, and we showed that SWT <200 m predicted mortality. However, inability to walk 200 m was not related to heart failure rehospitalization. Patients with inability to walk 200 m had significantly more heart failure hospitalizations in the past than those who could walk more than 200 m. It is well known that repeated hospitalizations lead to gradual loss of physical capacity and death. 23 Many patients who were unable to walk 200 m had repeated hospitalizations for heart failure before admission, and had likely reached the end stage of heart failure.
Nutritional status and physical capacity
Only the combined index including both GNRI and SWT was an independent predictor of all‐cause mortality in the multivariable Cox hazard analysis, after adjustment for other parameters reported to predict heart failure mortality in a previous study. 24 We showed that the combined index including both GNRI and SWT was a stronger predictor of the prognosis than either index alone. Group G (both nutritional status and physical capacity at discharge were good) showed the lowest mortality among the groups. It is interesting to speculate that achievement of a GNRI score ≥ 92 and the ability to walk 200 m at discharge may reduce mortality in these patients. Further prospective studies are needed to confirm these findings.
Limitations
First, this is a single‐centre study with a limited sample size. Second, our study population included only elderly patients with heart failure. Therefore, further research is needed to determine if results can be extended to a younger population. Third, we did not examine sex differences, which might have an impact on prognosis, particularly in elderly patients with heart failure with preserved EF. Fourth, this study was a retrospective and observational study. Thus, it is not clear whether interventions for nutrition status and physical capacity improve prognosis.
Conclusions
It was feasible to perform both GNRI and SWT in elderly patients with ADHF. The simple indicator including both nutritional status and physical capacity may predict 2 year mortality in elderly patients with ADHF.
Conflict of interest
Haruhiko Abe has received grants from Nippon Boehringer Ingelheim. Yasunori Ueda has received grants from Nihon Kohden. Masaaki Uematsu has received grants from Nippon Boehringer Ingelheim, Daiichi Sankyo Co Ltd. Yukihiro Koretsune has received remuneration from Daiichi Sankyo Co Ltd. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Funding
None.
Yasumura, K. , Abe, H. , Iida, Y. , Kato, T. , Nakamura, M. , Toriyama, C. , Nishida, H. , Idemoto, A. , Shinouchi, K. , Mishima, T. , Awata, M. , Date, M. , Ueda, Y. , Uematsu, M. , and Koretsune, Y. (2020) Prognostic impact of nutritional status and physical capacity in elderly patients with acute decompensated heart failure. ESC Heart Failure, 7: 1801–1808. 10.1002/ehf2.12743.
References
- 1. Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Miyata M, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Suzuki H, Ishida T, Takeishi Y. Impact of nutritional indices on mortality in patients with heart failure. Open Heart 2018; 5: e000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sze S, Zhang J, Pellicori P, Morgan D, Hoye A, Clark AL. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol 2017; 106: 533–541. [DOI] [PubMed] [Google Scholar]
- 3. Narumi T, Arimoto T, Funayama A, Kadowaki S, Otaki Y, Nishiyama S, Takahashi H, Shishido T, Miyashita T, Miyamoto T, Watanabe T, Kubota I. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J Cardiol 2013; 62: 307–313. [DOI] [PubMed] [Google Scholar]
- 4. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype; Cardiovascular Health Study Collaborative Research Group. Journals of gerontology Ser A Biol Sci Med Sci 2001; 56: M146–156. [DOI] [PubMed] [Google Scholar]
- 5. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 6. Yamada M, Arai H. Predictive value of frailty scores for healthy life expectancy in community‐dwelling older Japanese adults. J Am Med Dir Assoc 2015; 16: 1002.e7–1002.e11. [DOI] [PubMed] [Google Scholar]
- 7. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 8. Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric nutritional risk index: a new index for evaluating at‐risk elderly medical patients. Am J Clin Nutr 2005; 82: 777–783. [DOI] [PubMed] [Google Scholar]
- 9. Terminology and Diagnostic Criteria Committee of The Japan Society of Ultrasonics in Medicine . Standard measurement of cardiac function indexes. J Med Ultrason 2006; 33: 123–127. [DOI] [PubMed] [Google Scholar]
- 10. Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, Yamamoto K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J 2013; 77: 705–711. [DOI] [PubMed] [Google Scholar]
- 11. Kaneko H, Suzuki S, Goto M, Yuzawa Y, Arita T, Yagi N, Murata N, Kato Y, Kano H, Matsuno S, Otsuka T, Uejima T, Takai H, Oikawa Y, Kunihara T, Nagashima K, Kirigaya H, Sagara K, Sawada H, Aizawa T, Yajima J, Yamashita T. Geriatric nutritional risk index in hospitalized heart failure patients. Int J Cardiol 2015; 181: 213–215. [DOI] [PubMed] [Google Scholar]
- 12. JCS Joint Working Group . Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ J 2014; 78: 2022–2093. [DOI] [PubMed] [Google Scholar]
- 13. Leon AS, Franklin BA, Costa F, Balady GJ, Berra KA, Stewart KJ, Thompson PD, Williams MA, Lauer MS. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2005; 111: 369–376. [DOI] [PubMed] [Google Scholar]
- 14. Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, Froelicher VF, Leon AS, Piña IL, Rodney R, Simons‐Morton DA, Williams MA, Bazzarre T. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation 2001; 104: 1694–1740. [DOI] [PubMed] [Google Scholar]
- 15. Nishi I, Seo Y, Hamada Y, Yamamoto M, Ishizu T, Sugano A, Sato K, Sai S, Obara K, Suzuki S, Koike A, Aonuma K, Ieda M. Geriatric nutritional risk index predicts all‐cause deaths in heart failure with preserved ejection fraction. ESC Heart Failure 2019; 6: 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. White JV, Guenter P, Jensen G, Malone A, Schofield M. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Parenter Enteral Nutr 2012; 36: 275–283. [DOI] [PubMed] [Google Scholar]
- 17. Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, Muscaritoli M, Nyulasi I, Ockenga J, Schneider SM, de van der Schueren MA, Singer P. Diagnostic criteria for malnutrition ‐ an ESPEN consensus statement. Clin Nutr 2015; 34: 335–340. [DOI] [PubMed] [Google Scholar]
- 18. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar‐Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr 2008; 27: 793–799. [DOI] [PubMed] [Google Scholar]
- 19. Honda Y, Nagai T, Iwakami N, Sugano Y, Honda S, Okada A, Asaumi Y, Aiba T, Noguchi T, Kusano K, Ogawa H, Yasuda S, Anzai T, Investigators NDEF. Usefulness of geriatric nutritional risk index for assessing nutritional status and its prognostic impact in patients aged 65 years with acute heart failure. Am J Cardiol 2016; 118: 550–555. [DOI] [PubMed] [Google Scholar]
- 20. Stevenson LW, Steimle AE, Fonarow G, Kermani M, Kermani D, Hamilton MA, Moriguchi JD, Walden J, Tillisch JH, Drinkwater DC. Improvement in exercise capacity of candidates awaiting heart transplantation. J Am Coll Cardiol 1995; 25: 163–170. [DOI] [PubMed] [Google Scholar]
- 21. Forman DE, Fleg JL, Kitzman DW, Brawner CA, Swank AM, McKelvie RS, Clare RM, Ellis SJ, Dunlap ME, Bittner V. 6‐min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol 2012; 60: 2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curtis JP, Rathore SS, Wang Y, Krumholz HM. The association of 6‐minute walk performance and outcomes in stable outpatients with heart failure. J Card Fail 2004; 10: 9–14. [PubMed] [Google Scholar]
- 23. Lunney JR, Lynn J, Hogan C. Profiles of older medicare decedents. J Am Geriatr Soc 2002; 50: 1108–1112. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki S, Yoshihisa A, Sato Y, Kanno Y, Watanabe S, Abe S, Sato T, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Ishida T, Takeishi Y. Clinical significance of Get with the Guidelines‐Heart Failure risk score in patients with chronic heart failure after hospitalization. J Am Heart Assoc 2018; 7: e008316. [DOI] [PMC free article] [PubMed] [Google Scholar]


