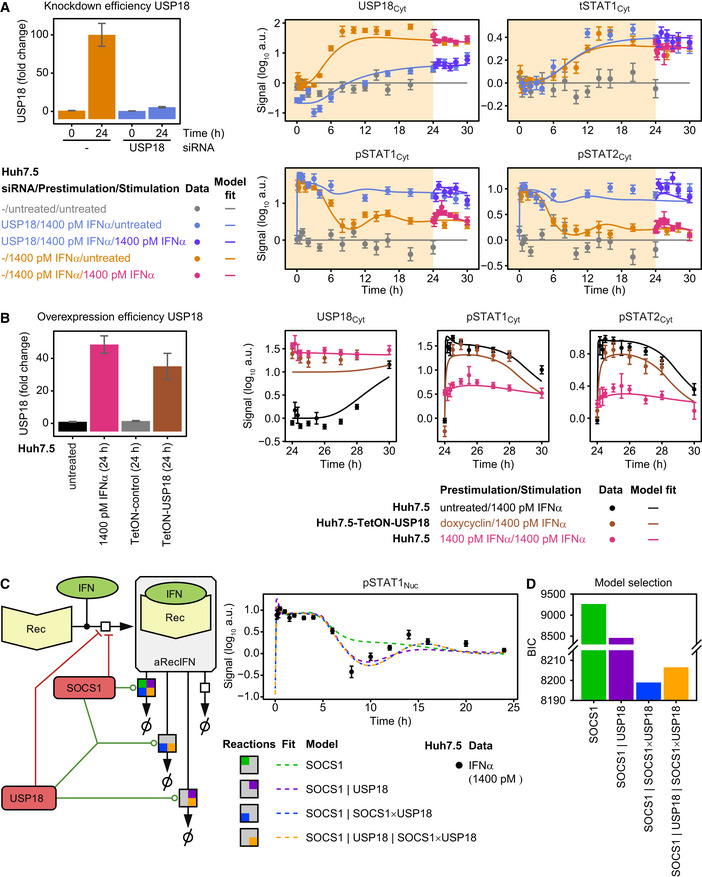
Knockdown of USP18 results in sustained signaling. USP18 knockdown efficiency determined by quantitative immunoblotting of cytoplasmic lysates of Huh7.5 transfected with USP18 siRNA relative to parental Huh7.5 cells and Huh7.5 cells transfected with non‐targeting siRNA. Cells were stimulated with 1,400 pM IFNα for 24 h. Error bars represent 1σ confidence intervals estimated from biological replicates (N = 6 to N = 10) (left). Model fit and experimental data of Huh7.5 cells transfected with control siRNA or USP18 siRNA are shown. Cells were transfected with USP18 siRNA or control siRNA 1 day prior to growth factor‐depletion. Next, cells were prestimulated with 1,400 pM IFNα (yellow background) and stimulated with 1,400 pM IFNα at 24 h or untreated (white background). IFNα‐induced phosphorylation of STAT1 and STAT2 and induction of USP18 and tSTAT1, comprising both phosphorylated and unphosphorylated STAT1 protein, are displayed. Experimental data were obtained by quantitative immunoblotting using chemiluminescence and CCD camera‐based device (ImageQuant). For model purposes, data in control siRNA and untransfected Huh7.5 are combined to one condition. Data from multiple time courses scaled together are displayed as filled circles with errors representing 1σ confidence intervals estimated from biological replicates (N = 2 to N = 10) using a combined scaling and error model. Lines represent model trajectories.
Overexpression of Huh7.5 is not sufficient to explain desensitization. Induced expression of USP18 after treatment of Huh7.5‐TetON‐USP18 and Huh7.5‐TetON‐control cells with doxycycline for 24 h in comparison with parental Huh7.5 cells treated with 1,400 pM IFNα for 24 h. Analysis was performed by quantitative immunoblotting. Error bars represent 1σ confidence intervals estimated from biological replicates (N = 3 to N = 5) (left). Model fits and experimental data of Huh7.5‐TetON‐USP18 treated with doxycycline for 24 h and stimulated with 1,400 pM IFNα or parental Huh7.5 cells prestimulated with 0 or 1,400 pM IFNα and stimulated with 1,400 pM IFNα after 24 h are shown. The dynamics of IFNα‐induced phosphorylation of STAT1 and STAT2 and induction of USP18 are depicted. Experimental data were obtained by quantitative immunoblotting using chemiluminescence and CCD camera‐based device (ImageQuant). For modeling purposes, data from Huh7.5‐TetON empty vector control and untransduced Huh7.5 are combined to one condition. Data are displayed as filled circles with errors representing 1σ confidence intervals estimated from biological replicates (N = 3 to N = 4) using a combined scaling and error model. Line represents model trajectories.
Scheme of possible mechanisms for SOCS1‐ and USP18‐induced receptor degradation. Models with different structures concerning SOCS1‐ and USP18‐catalyzed degradation of active receptor complexes (aRecIFN) were tested. Vertical lines denote separate enzymatic reactions, multiplication sign denotes cooperative enzymatic reactions. For each of the four different model structures, the resulting model trajectories of the best fit of pSTAT1 in the nucleus are exemplarily shown as dashed lines. Data are displayed as filled circles with errors representing 1σ confidence intervals estimated from biological replicates (N = 1 to N = 38).
Both SOCS1‐ and SOCS1:USP18‐induced receptor degradation is required. Bayesian information criterion (BIC) was used to evaluate goodness‐of‐fit for the four different models shown in (C). The model “SOCS1 | SOCS1 × USP18” that shows the best performance comprises degradation of the active receptor complexes by both SOCS1 and a SOCS1:USP18 complex.