Abstract
A new type of biodegradable film was formulated and characterized when based on the water-soluble-phase of Persian gum (SPG). The edible film was formulated optimally by using different concentrations of SPG (2.0, 2.5, 3.0, 3.5 and 4%) and glycerol as plasticizer (25, 35, and 35% based on dried SPG). Further examinations involved evaluating the manufactured films in terms of the barrier and physical properties, mechanical qualities, optical indices, microstructural properties and Fourier transform. The results showed that the increase in SPG and plasticizer content caused increases in thickness, moisture uptake, water vapor permeability and density of films (p < 0.05). Water solubility increased in response to higher concentrations of glycerol but decreased by higher amounts of dry matter (p < 0.05). The highest levels of the tensile strength (59.95%) and elongation at break (40.3 MPa) were obtained by SPG (3.5%) + 35% glycerol treatment. The L*, a* and opacity values decreased, while there was an increase in the b* value, as a result of increasing the plasticizer content (p < 0.05). A reduction occurred in the L* value of films, while the a* and b* values increased when using higher amounts of dry matter (p < 0.05). By analyzing the samples with field emission scanning electron microscopy, no cracks were observed on films when the contents of glycerol and dry matter were higher than 30% and 2.5%, respectively. The findings demonstrated that creating edible films from SPG can be an effective approach to the production of edible films.
Keywords: Persian gum, Edible films, Glycerol, Plasticizer, Food packaging
Introduction
The safety of non-biodegradable petroleum polymers is debated in the food industry (Soukoulis et al. 2016). The concerns over environmental threats, toxicity, high expenses, and sufficient availability have encouraged researchers to turn to biodegradable coatings and edible packaging films (Daza et al. 2018; Nafchi et al. 2017). Owing to their safety and range of sources, the naturalness of biopolymers carries substantial advantages over synthetic materials when using them in packaging, biomedical purposes and pharmaceuticals. However, the functional properties of materials that are commonly used as synthetics can pose several drawbacks, including the issues of availability and cost (Liang and Wang 2018). Accordingly, there is an ongoing quest to explore the newness of biological sources for food applications. Several compounds that constitute the chemical structure of biopolymers in making edible films and coatings include lipids, proteins, polysaccharides and their derivatives, the majority of which can be obtained from animals, plants, and microorganisms (Zhang et al. 2016). In making edible films, the provision of strength, compatibility and stability are of utmost importance, and polysaccharides are capable of giving edible films such desirable qualities (Sadeghi-Varkani et al. 2018). Many kinds of polysaccharides have been used recently to develop edible films, including Ghatti gum (Zhang et al. 2016), Artemisia sphaerocephala Krasch. gum (Liang and Wang 2018), Alyssum homolocarpum seed gum (Nafchi et al. 2017), Balangu seed (Sadeghi-Varkani et al. 2018), reinforced cassava starch (Iamareerat et al. 2018), and salep (Ekrami and Emam-jomeh 2014). Nonetheless, the brittleness of most polysaccharide-based films makes them prone to cracking in the course of their usage and storage. Polysaccharide films can be given more flexibility when, for instance, polyols such as glycerol are incorporated into their structures to serve as plasticizers (Ploypetchara and Gohtani 2018; Tong et al. 2011; Ekrami and Emam-jomeh 2014). The plasticizers, via weakening the biofilms’ molecular interactions, lead to greater flexibility in films (Farhan and Hani 2017, Nur-Hanani et al., 2013). The type of biopolymer and its concentration, besides the concentration of plasticizers, can be largely capable of improving the quality of films in terms of their physical and mechanical properties (Daza et al. 2018; Chevalier et al. 2018; Dick et al. 2015).
The Persian gum (PG) is a natural biopolymer that comes from the tree of Amygdalus scoparia Spach. (Synonym: Prunus scoparia Spach.) This species can be found in the forests of Irani–Tourani regions (Heidari et al. 2008). The PG is relatively cheap compared to other natural hydrocolloids, and this makes it a suitable substitute (Raoufi et al. 2017). Based on the dry weight, PG is composed of about 95% polysaccharides—mainly of galactopyranosyl and arabinofuranosyl units (Dabestani et al. 2018). About 25–30% w/w of PG can usually be dissolved in water (Dabestani et al. 2018), thereby being a suitable compound for the creation of film-forming solutions.
A review of the available literature shows that no specific research has dealt with the production of edible films based on PGs so far. Preliminary results of our studies have shown that the soluble portion of PG (SPG) is capable of yielding films that appear physically good, but have the disadvantage of being brittle and thus inappropriate for packaging. The current research aims to develop a biodegradable film that is based on PG, while applying several concentrations of glycerol and SPG. It is hypothesized that these edible films have unique features in terms of their physical and mechanical properties, in addition to their role as a barrier. Their optical and micro-structural characteristics are also studied herein.
Material and method
Preparation of films
Dried PG granules were obtained from a reputable herbal market. The PG was transferred to the laboratory and authenticated at the Department of Horticulture Science, Faculty of Agriculture, University of Zabol, Zabol, Iran. The SPG was made, according to the method used by Mohammadi et al. (2016). For this purpose, the PG granule with white color was powdered using a laboratory mill device and was sieved in a shaker device (Retsch, USA) via a sieve (ASTM standard E-11) with a mesh number of 100. One g of PG powder was added to 100 mL of sterilized distilled water and was maintained inside a stirred glass vessel at 16 °C for 24 h. The suspension was centrifuged (Eppendorf 5810, Germany) for 20 min at 3500 g. After removing the insoluble phase, the purified PG solution was freeze-dried (Armfield, UK), vacuum-packed and then kept at 4 °C for 72 h until further use. The film formation solution (FFS) was prepared by adding dry powder (at 2.0, 2.5, 3.0, 3.5 and 4.0 g) to 100 ml sterilized distilled water which was stirred for 1 h at 40 °C. Then, glycerol at 25, 30 and 35% (w/w based on dried SPG) was added to the mixture as a plasticizer. Accordingly, the pH of FFS was adjusted to 7.0 and was homogenized in a rotor–stator ultraturrax (DI25, Germany) at 13,500 rpm for 4 min. Then, the solutions were heated for 15 min at 85 °C, before being quickly cooled down to 5 °C so as to kill undesirable microbes. The degasified FFS was made by a vacuum pump (Jencons, England). SPG films were made by casting 25 ml of FFS in plastic petri dishes (10 cm diameter). They were left to dry at 30 °C and at 40% RH for 48 h. The samples were conditioned for 4 days at 24 °C in a desiccator which was filled with a saturated solution of Mg(NO3)2 to provide 52% RH. The conditioning was a necessary step prior to the characterization of films.
Characterization of films
Scanning electron microscopy (SEM)
First, the samples were treated with liquid nitrogen and, then, they were mounted onto aluminum stubs via tapes that were double-sided. A thin layer of gold was coated onto the films, and then cross-section images were obtained therefrom by an electron microscope (EM3200, KYKY, USA) with an acceleration voltage of 26 kV.
Thickness and density
A digital micrometer (Mitutoyo CO, Japan) was used for measuring the thickness of films, and the density was calculated according to a method used by Tajik et al. (2013).
Moisture content
The film samples (with the dimensions of 3 × 3 cm) contained various levels of moisture content which were measured gravimetrically (Sadeghi-Varkani et al. 2018).
Moisture uptake of films
The films were cut into 2.0 cm × 4.0 cm dimensions to make samples measurable in terms of their moisture uptake. Depending on the conditions, the samples displayed different behaviors of moisture uptake, the values of which were measured by a K2SO4-saturated solution when placed inside a desiccator (48 h, 25 °C RH = 97%) (Nafchi et al. 2017).
Measurement of film solubility in water
Film samples (2 × 4 cm) were dried at 60 °C for 24 h, and their solubility was measured according to a previously used procedure (Singh et al. 2015).
Water vapor permeability (WVP)
The ASTM method E96-95 was followed in measuring the WVP, as described by Beristain-Bauza et al. (2016).
Oxygen permeability
By reacting with H2O at 90% RH, iron powder was reduced to Fe (OH)2 while the reaction was facilitated by activated carbon and NaCl (Zhang et al. 2016) so as to measure oxygen permeability. A bottle containing 3.0 g of deoxidizing agent was covered by film samples and put into a desiccator which had a saturated solution of barium chloride (RH = 90%, 25 °C, 48 h). Changes in the weight of the bottle were recorded and the OP was calculated (g/m2 s).
Mechanical properties
A texture analyzer was employed to measure the elastic modulus (EM), the elongation at break (EB%) and tensile strength (TS). Before the test, the moisture of film samples was balanced in an atmosphere of 52% RH at 25 °C. Then, the films were divided into rectangular shapes (2.5 × 10.0 cm) in accordance with the Standard ASTM D-882 (ASTM 2001). The cross-head speed operated at 5.0 cm per minute, and the grip separation was prepared at 5.0 cm (Vargas et al. 2011).
Optical properties
The films were cut into rectangular shapes (0.7 × 1.5 cm2), before reading the absorbance value at 600 nm by a UV–VIS spectrophotometer (Haq et al. 2016). The film samples were characterized in terms of color by using a tristimulus colorimetry which measured the redness/greenness (a*), lightness/brightness (L*) and yellowness/blueness (b*), besides employing the Minolta equipment (Model CR-300, Minolta Co., New York) (Farhan and Hani 2017).
Fourier transform infrared spectroscopy (FTIR)
Samples were prepared for spectroscopy. Potassium bromide powder (KBr) was mixed with powdered samples, and then pressing the mixture yielded small pellets. An infrared spectrometer chamber was used for measuring light transmission (Transmittance %) versus wave number (400–4000 cm−1) (Mohammadi et al. 2016).
Statistical analyses
A factorial type of experiment was used in association with a completely randomized design (CRD). The results were processed by SAS v. 9.1. Each experiment was performed at least in triplicate. The mean values pertaining to the physical and mechanical data were compared using Duncan’s test. The differences were considered significant at p value < 0.05.
Results and discussion
SPG film formulation and morphological characterization
Using proper concentrations of plasticizers and appropriate amounts of dry materials led to the production of film samples that could be handled well in the process of being created and also after it. The absence of plasticizers in film-forming solutions, or even their presence at concentrations lower than 25% (w/w based on dried SPG), led to the production of brittle films. Handling such films was difficult, especially when removing them from the casting plates. Raising the amount of glycerol to concentrations of more than 35% (w/w) caused softening and stickiness in SPG films. Plasticizers are essential to the structural stability of biochemical films as they provide sufficient amounts of molecular mobility and intermolecular spacing among the polymer chains, thereby granting biopolymer films greater flexibility. This happens while intermolecular forces become weaker between chain-to-chain interactions of polymers (Nur-Hanani et al. 2013). Moreover, the films could be peeled off conveniently from the casting plates when there was a minimum of 2.0% (w/v) SPG in the film-forming solutions. However, high amounts of dried SPG, i.e. over 4.0% (w/v), led to the production of films that were hard to peel off from the casting plate because of an acquired gummy, viscous quality.
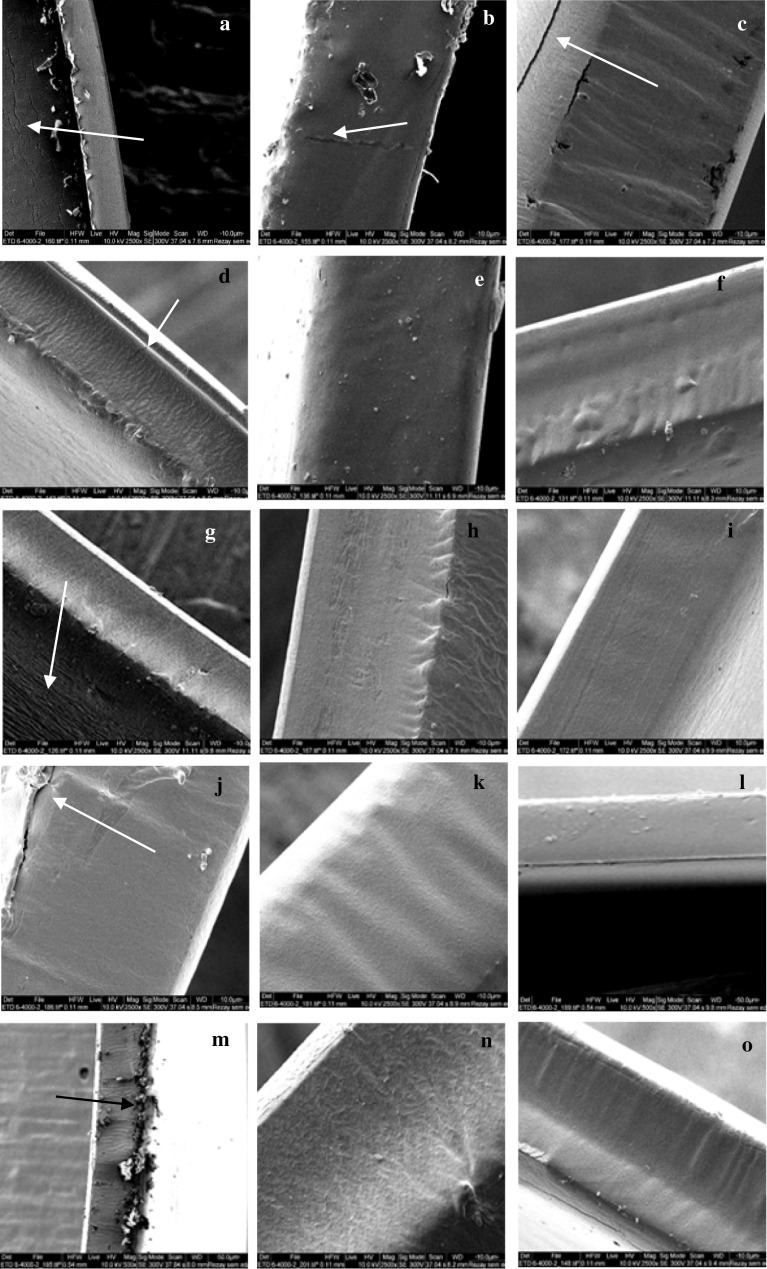
Plasticizing the SPG-based FFS of all film samples with various amounts of dry matter (2.0–4.0%) and variable concentrations of glycerol (25–35%) caused the films to acquire a good appearance and a smooth, undamaged surface from a microscopic perspective, besides having flexibility and transparency. Incorporating different concentrations of glycerol and dry matter into SPG films led to their acquisition of different microstructures (Fig. 1).
Fig. 1.
Scanning electron microscopy (2500 ×) of the films. Samples containing 2.0% SPG and 25%, 30% and 35% Gly named a–c, respectively; Samples containing 3.0% SPG and 25%, 30% and 35% Gly named d–f, respectively; Samples containing 3.0% SPG and 25%, 30% and 35% Gly named g–i, respectively; Samples containing 3.5% SPG and 25%, 30% and 35% Gly named j–l, respectively; Samples containing 4.0% SPG and 25%, 30% and 35% Gly named m–o, respectively; SPG: Water soluble phase of persian gum, Gly: glycerol
Cracks are evident on the surface-layer and cross-sectional samples of films which contained 2% dry matter (a, b and c), and also on those which contained 25% glycerol (d, g, and j). Many particles were seen on the edge of films which contained 4.0% SPG and 35% glycerol (Fig. 1m). Other samples of films showed complete homogeneity and their structural differences remained negligible.
Physical properties of SPG films
The amounts and concentrations of SPG and glycerol affected the physical properties of SPG films (Table 1). The thickness of films measured between 0.049 mm and 0.113 mm. Where the amount of dried SPG was kept constant, higher concentrations of glycerol led to significantly thicker films (p < 0.05). This may be due to the fact that glycerol molecules tend to interact with the film-forming polymer, thereby enlarging the spaces within and between the polymer—hence thicker films (Arham et al. 2016). Similarly, while keeping the glycerol at a fixed level, providing higher amounts of biopolymer led to the production of significantly thicker films (p < 0.05). A similar trend of change was observed in the amount of moisture content (as it increased from 15.21 to 30.26%) and in moisture uptake by the films (from 33.0 to 75.3 g/g dried film) (Table 1). These results accord closely with previous reports on different levels of chitosan and glycerol concentrations in chitosan-based films (Singh et al. 2015). SPG usually shows high amounts of hydrophilicity, which partly explains the latter observation, while 12 g of water can be imbibed by one gram of PG on average (Dabestani et al. 2018). The moisture content of films can undergo slight amounts of increase due to the attachment of water molecules by hydrogen bonds as provided by hydroxyl groups in the biopolymer. Furthermore, glycerol-water bonds and glycerol-polymer bonds can grow numerous in response to higher concentrations of glycerol in the FFS (Osés et al. 2009). This ultimately leads to the increase of moisture content in films and their thickening, which is primarily because the biopolymer structure would be more likely to absorb water molecules in such circumstances. These observations are similar to previous ones reported on edible films that were tested when based on Balangu Seed Mucilage (Sadeghi-Varkani et al. 2018).
Table 1.
Thickness, density, moisture content, moisture uptake, water solubility, and contact angle of Persian Gum based films at different concentrations of dry matter and glycerol
| Film composition | Thickness (mm) | Moisture content (%) | Moisture uptake (g/g dried film) | Density (g/cm3) | Water solubility (%) | WVP (×1011 g/m2 s) | O2P (×10−3 g/m2 s) |
|---|---|---|---|---|---|---|---|
| SPG (2.0%) + 25% Gly | 0.049 ± 0.002k | 15.21 ± 0.22n | 33.0 ± 3.4j | 0.81 ± 0.02g | 80.0 ± 1.4c | 3.9 ± 0.12j | 3.6 ± 0.04d |
| SPG (2.0%) + 30% Gly | 0.052 ± 0.001jk | 16.48 ± 0.18m | 46.3 ± 3.7gh | 0.80 ± 0.01g | 84.3 ± 0.8b | 4.5 ± 0.16i | 5.7 ± 0.07b |
| SPG (2.0%) + 35% Gly | 0.056 ± 0.001ij | 17.66 ± 0.15l | 52.6 ± 2.9def | 0.78 ± 0.01g | 89.6 ± 0.9a | 5.5 ± 0.154g | 6.4 ± 0.11a |
| PG (2.5%) + 25% Gly | 0.060 ± 0.003i | 19.04 ± 0.09k | 37.7 ± 1.9i | 1.06 ± 0.03e | 72.7 ± 0.9e | 4.6 ± 0.17i | 2.4 ± 0.06j |
| SPG (2.5%) + 30% Gly | 0.066 ± 0.002h | 20.33 ± 0.26j | 50.6 ± 2.7efg | 1.03 ± 0.01ef | 75.6 ± 0.7d | 5.1 ± 0.12h | 2.6 ± 0.03hi |
| SPG (2.5%) + 35% Gly | 0.071 ± 0.002g | 21.47 ± 0.29i | 57.2 ± 3.3cd | 1.01 ± 0.02f | 79.2 ± 1.3c | 6.2 ± 1.0f | 2.7 ± 0.10h |
| SPG (3.0%) + 25% Gly | 0.080 ± 0.003f | 22.85 ± 0.17h | 43.8 ± 2.4h | 1.18 ± 0.02bc | 64.4 ± 1.1h | 6.3 ± 0.11f | 2.5 ± 0.12ij |
| SPG (3.0%) + 30% Gly | 0.088 ± 0.001e | 23.53 ± 0.31g | 53.9 ± 2.1cde | 1.17 ± 0.02cd | 67.8 ± 1.4g | 6.9 ± 0.14e | 3.1 ± 0.05f |
| SPG (3.0%) + 35% Gly | 0.091 ± 0.004de | 24.81 ± 0.14f | 58.3 ± 2.6c | 1.14 ± 0.01d | 69.9 ± 0.7f | 7.4 ± 0.13d | 3.6 ± 0.04d |
| SPG (3.5%) + 25% Gly | 0.093 ± 0.004d | 26.01 ± 0.12e | 48.1 ± 1.8fgh | 1.21 ± 0.03ab | 57.2 ± 1.1j | 6.7 ± 0.20e | 3.2 ± 0.02ef |
| SPG (3.5%) + 30% Gly | 0.095 ± 0.004d | 26.98 ± 0.28d | 56.2 ± 2.3cd | 1.18 ± 0.02bc | 59.6 ± 1.3i | 7.5 ± 0.15cd | 3.5 ± 0.05d |
| SPG (3.5%) + 35% Gly | 0.105 ± 0.002c | 28.01 ± 0.16c | 59.1 ± 2.5bc | 1.16 ± 0.01cd | 64.6 ± 1.6h | 8.1 ± 0.19b | 4.1 ± 0.11c |
| SPG (4.0%) + 25% Gly | 0.107 ± 0.004bc | 29.44 ± 0.25b | 52.5 ± 3.8def | 1.24 ± 0.03a | 52.3 ± 1.0k | 7.7 ± 0.23c | 3.3 ± 0.05e |
| SPG (4.0%) + 30% Gly | 0.110 ± 0.001ab | 30.05 ± 0.18a | 63.6 ± 2.9b | 1.19 ± 0.01bc | 57.6 ± 1.2j | 8.3 ± 0.11b | 3.2 ± 0.04ef |
| SPG (4.0%) + 35% Gly | 0.113 ± 0.001a | 30.36 ± 0.21a | 75.3 ± 3.0a | 1.18 ± 0.01bc | 61.5 ± 1.8i | 8.5 ± 0.21a | 2.9 ± 0.03g |
Data in columns with different superscripts are significantly different (p < 0.05). Data are means ± standard deviation. SPG: dried Soluble fractionof persian gum Gly: Glycerol
Here, the density values of films varied (0.78–1.21 g/cm3) depending on the composition of their film-forming solution (Table 1). With a fixed level of glycerol, the increase in dry matter had a significant effect on the increase in density. However, increasing the concentration of glycerol slightly reduced the density of films. Similar observations were made by Singh et al. (2015).
Water is generally a good substrate for the solubility of edible films. This feature can be managed in medicinal applications where biochemical delivery systems and their mechanisms are important. In such circumstances, edible films function as the outer layer of capsules in which the drug is encased. After being exposed to water for a certain amount of time, the edible film is dissolved safely and the drug is released into the stomach. (Nafchi et al. 2017). The SPG edible films are not exactly the same in terms of water solubility (Table 1). Clearly, the rate of increase in the water solubility of the fabricated films—with respect to the glycerol level—is inversely related to the dried gum content of the films (p < 0.05). Previous research on edible films, based on agar, yielded similar results (Arham et al. 2016). The occurrence of crystallization between chains of polymers and the networks of cross-links therein determine the degree of water solubility in polymers. As explained earlier, the hydrophilic property of edible films can be intensified by bonds between polymers and glycerol in films that are infused with glycerol, thereby leading to a greater capacity for water solubility (Liu and Yao 2001).
As can be seen in Table 1, when glycerol and/or dry matter concentrations increase, the WVP improves significantly (p < 0.05). In a previous study, similar observations were made when the concentration of Balangu seed mucilage increased from 1.2 to 1.6% (Sadeghi-Varkani et al. 2018). This is reflected on how hydrophilicity is increased by the effect of plasticizer inside SPG-based films, as explained in the previous section. The WVP values of SPG-based films (3.9–8.5 × 1011 g/s m Pa) can be compared with those of gum ghatti (0.71–16.62 × 1011 g/s m Pa) (Zhang et al. 2016), Alyssum homolocarpum gum (4.93–6.91 × 1011 g/s m Pa) (Nafchi et al. 2017) and agar (9.28 × 10 11 g/s m Pa) (Wang et al. 2018).
The oxidation of food is significantly dependent on the availability of oxygen, and food preservation relies partly on the O2P of materials that are used in food packaging (Sothornvit and Pitak 2007). Usually, there are changes in the trends of O2P in SPG edible films (Table 1). The highest value of O2P (6.4 × 10 −3 g/m2 s) was obtained by SPG films which contained 2% dry material and 35% glycerol (p < 0.05). Higher values of O2P were obtained by increasing the amount of glycerol from 25 to 35%, despite keeping the concentration of dry materials constant. Nonetheless, increasing the glycerol concentration in the presence of 4% dry matter caused reductions in the O2P value. It has been stated in the available literature that such an observation could be explained by the changes that occur to the mobility of biopolymer-chains in the film matrix since hydrogen bonds are abundantly available there. This improves the molecular ability to bar oxygen and other non-polar compounds from passing through the film (Zhang et al. 2016). Additionally, depth cracking on cross-sectional and surface areas of film samples (Fig. 1a–c) can increase the permeability of oxygen. Furthermore, the O2P values of SPG-based films (2.4–6.4 × 10−3 g/m2 s) are comparable to those observed previously in gum ghatti (1.0–1.3 × 10 −3 g/m2 s) (Zhang et al. 2016).
Mechanical properties of SPG films
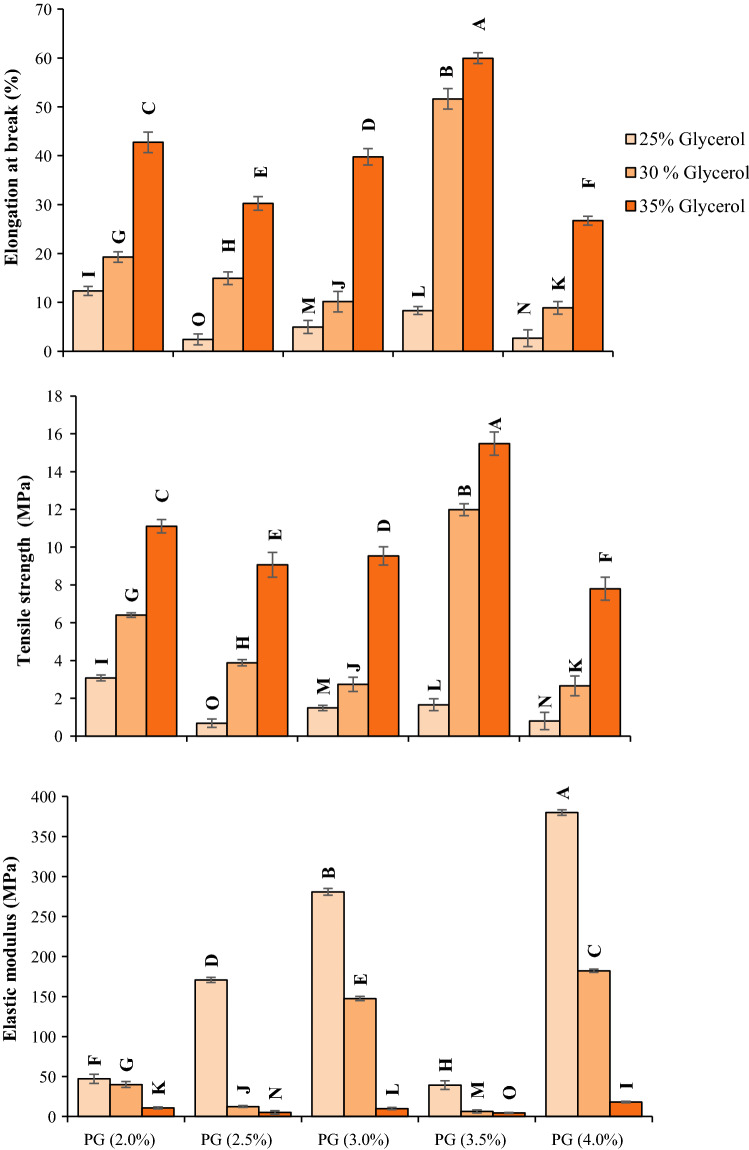
The flexibility, strength, and stiffness of films can be measured by the elongation at break values (EB%), tensile strength (TS), and elastic modulus (EM), respectively. In other words, these parameters can be used for characterizing mechanical properties in films. The concentrations of dry matter and plasticizer can significantly affect SPG-based films, especially when considering their mechanical properties (Fig. 2). Using the combination of SPG (3.5%) + 35% glycerol, the film acquired the highest TS (40.3 MPa) and EB (59.95%) values and the lowest EM value (4.63 MPa). At a fixed concentration of dry matter, the provision of higher amounts of plasticizer caused significant increases in the EB and TS values of SPG films, whereas the EM value decreased substantially. The propensity for hydrogen bonding between plasticizer molecules and matrix chains could define how strongly the polymer connects to the plasticizer (Farhan and Hani 2017). As a plasticizer, glycerol is capable of absorbing high amounts of moisture. Furthermore, water itself has the ability to add to the plasticizing effect (Dick et al. 2015). Accordingly, the provision of more glycerol in the film-forming solutions improved the flexibility of SPG films, but at the cost of reducing their mechanical resistance. The concentration of plasticity and its effect on the mechanical properties of films have been widely discussed in the scientific literature (Ghasemlou et al. 2013). The TS values of SPG-based films (0.68–15.48 MPa) are comparable to those of Alyssum homolocarpum seed gum (11.87–19.39 MPa) (Nafchi et al. 2017), agar (5.3–19.8 MPa) (Arham et al. 2016) and gum ghatti (3.5–54.2 MPa) (Zhang et al. 2016). Furthermore, the EB values of SPG-based films (2.44–59.95%), as recorded in this research, are higher than the EB values in Alyssum homolocarpum seed gum (about 23.2–42.1%) (Nafchi et al. 2017), agar (15.3–27.8%) (Arham et al. 2016) and gum ghatti (about 3.2–34.2 MPa) (Zhang et al. 2016).
Fig. 2.
Mechanical properties of persian gum based films at different concentrations of dry matter and glycerol. Data in columns with different superscripts are significantly different (p < 0.05). Data are means ± standard deviation
Optical properties
Color and opacity are two important factors that shape the ultimate decision made by consumers in using edible and biodegradable films (Ekrami and Emam-jomeh 2014; Bourtoom 2008). The SPG films appeared to have different colors when analyzing them by the b*, a* and L* factors (Table 2). It was observed that increasing the concentrations of plasticizer and dry matter made the films show lower values of L* and a *, thereby indicating that the films appear darker and more red (p < 0.05). Increasing the amounts of glycerol and dry matter caused a significant rise in the b* value (p < 0.05). This denotes that the films are inclined towards yellowness. This may be a result of new bond formations between glycerol and polymer chains in the biofilm matrix which tend to alter the color index (Ekrami and Emam-jomeh 2014).
Table 2.
Optical properties of persian gum based films at different concentrations of dry matter and glycerol
| Film composition | L * | a * | b * | Opacity |
|---|---|---|---|---|
| SPG (2.0%) + 25% Gly | 92.83 ± 0.13a | 1.25 ± 0.04def | − 1.70 ± 0.09i | 0.86 ± 0.03a |
| SPG (2.0%) + 30% Gly | 92.41 ± 0.11e | 1.18 ± 0.09ef | − 1.52 ± 0.02h | 0.73 ± 0.03c |
| SPG (2.0%) + 35% Gly | 92.25 ± 0.05h | 1.15 ± 0.05f | − 1.23 ± 0.07g | 0.67 ± 0.04d |
| SPG (2.5%) + 25% Gly | 92.75 ± 0.08b | 1.31 ± 0.09h | − 1.28 ± 0.03g | 0.74 ± 0.03bc |
| SPG (2.5%) + 30% Gly | 92.64 ± 0.18c | 1.24 ± 0.06k | − 1.04 ± 0.07f | 0.71 ± 0.03cd |
| SPG (2.5%) + 35% Gly | 92.48 ± 0.09d | 1.22 ± 0.08l | − 0.98 ± 0.09def | 0.52 ± 0.03ef |
| SPG (3.0%) + 25% Gly | 92.31 ± 0.17f | 1.43 ± 0.11d | − 1.00 ± 0.13ef | 0.78 ± 0.04b |
| SPG (3.0%) + 30% Gly | 92.26 ± 0.11g | 1.32 ± 0.08g | − 0.97 ± 0.08def | 0.54 ± 0.02e |
| SPG (3.0%) + 35% Gly | 91.88 ± 0.02j | 1.26 ± 0.03i | − 0.89 ± 0.02abc | 0.47 ± 0.03f |
| SPG (3.5%) + 25% Gly | 92.01 ± 0.15i | 1.48 ± 0.06b | − 0.95 ± 0.04def | 0.76 ± 0.04bc |
| SPG (3.5%) + 30% Gly | 91.86 ± 0.17k | 1.35 ± 0.09e | − 0.84 ± 0.05bcd | 0.73 ± 0.02c |
| SPG (3.5%) + 35% Gly | 91.74 ± 0.09l | 1.32 ± 0.07g | − 0.73 ± 0.09ab | 0.57 ± 0.04e |
| SPG (4.0%) + 25% Gly | 91.13 ± 0.18m | 1.52 ± 0.04a | − 0.89 ± 0.03cde | 0.84 ± 0.04ab |
| SPG (4.0%) + 30% Gly | 90.89 ± 0.07n | 1.47 ± 0.07c | − 0.76 ± 0.08abc | 0.79 ± 0.03b |
| SPG (4.0%) + 35% Gly | 90.44 ± 0.12o | 1.34 ± 0.13f | − 0.69 ± 0.03 a | 0.77 ± 0.04bc |
Data in columns with different superscripts are significantly different (p < 0.05). Data are means ± standard deviation. SPG: dried Soluble fraction of persian gum Gly: Glycerol
Consumers generally have the tendency to favor opacity or transparency in selecting packaging materials. In this research, there were different levels of opacity in the films (Table 2). All plasticized films showed low values of opacity (i.e. less than 1), suggesting high levels of transparency in the appearance of films. The highest values of opacity were obtained in the films made of SPG (2.0%) + 25% glycerol and SPG (4.0%) + 25% glycerol, whereas lower values were obtained in the films made of SPG (3.0%) + 35% glycerol (p < 0.05). The results showed that the opacity of films can be improved by adding plasticizers to the SPG matrix. This is mainly because the addition of glycerol can expand intermolecular spacing and enhance the mobility of polymer chains in the SPG matrix (Nafchi et al. 2017). Increasing the amount of SPG had different effects on the opacity of films. In fact, the surface morphology of films is a determinant factor for such variations in opacity because the specular reflectance can be affected by the homogeneity of the film matrix at the air–film interface. In other words, higher levels of homogeneity provide lower levels of opacity (Kibar and Ferhunde 2017). These arguments are approved by the SEM results obtained in the current study (Fig. 1).
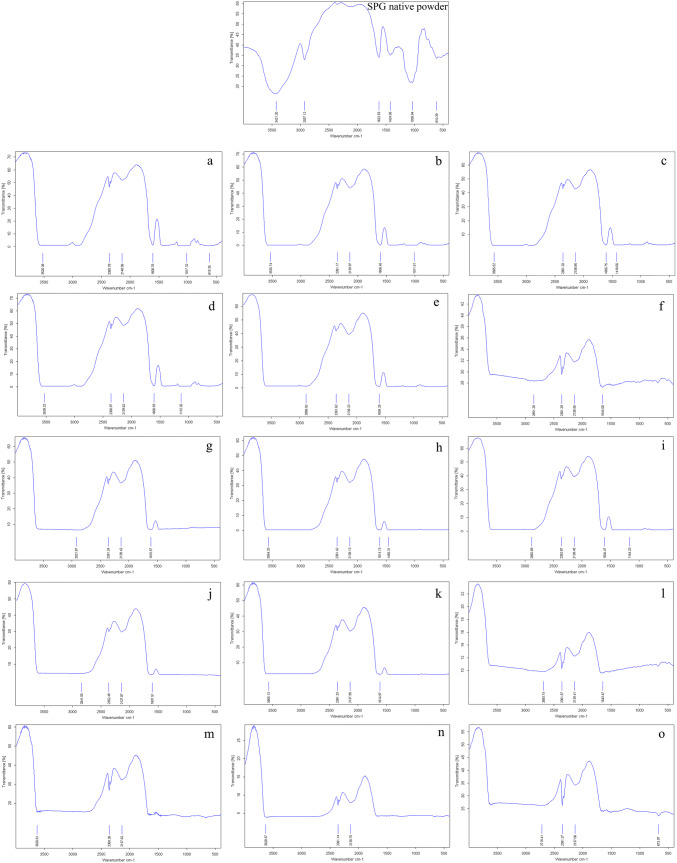
Fourier transform infrared spectroscopy (FTIR)
The functional groups of carbohydrates are primarily described by FTIR spectra. In this regard, there are variations in the spectra of SPG-control and SPG-films (Fig. 3). In the spectra of SPG (Fig. 3a), the presence of COOH or the bonding vibration of CH3 represent the carboxylate groups of uronic acid residues which trigger absorption at around 1424 cm−1. Carboxyl groups are likely to produce anhydride components, thereby causing the absorption to peak at 1622 cm−1. Asymmetric and symmetric patterns of stretching between C–H bonds are known to prompt various other distinguishable peaks (2927 cm−1), even though such a range of absorption can also be caused by asymmetric CH2 functional groups and the overlap between double bonds in O–H. Furthermore, peaks at 1035 cm−1 can be attributed to C–O stretching. Nonetheless, there are no clear explanations for peaks that range between 2138 and 3421 cm−1 (Abbasi 2017a, b). In the current research, the effect of glycerol as a plasticizer was clearly noticeable in films, as compared to the SPG.
Fig. 3.
FT-IR spectrum of SPG powder and prepared films. Samples containing 2.0% SPG and 25%, 30% and 35% Gly named a–c, respectively; Samples containing 3.0% SPG and 25%, 30% and 35% Gly named d–f, respectively; Samples containing 3.0% SPG and 25%, 30% and 35% Gly named g–i, respectively; Samples containing 3.5% SPG and 25%, 30% and 35% Gly named j–l, respectively; Samples containing 4.0% SPG and 25%, 30% and 35% Gly named m, n and o, respectively; SPG: Water soluble phase of persian gum, Gly: glycerol
Conclusion
This research led to the first report of its kind on using the soluble phase of Persian gum (SPG) as a biopolymer-base for the production of edible films. The flexibility and homogeneity of the films are largely influenced by the concentrations of glycerol and SPG. Testing a variation of these concentrations on the film-forming solutions led to the creation of a diverse range of SPG films with different physicochemical and fundamental properties. To achieve the highest degrees of extensibility, elastic modulus and the lowest degree of opacity, it is recommended here that the combination of 3.0% SPG content and 35% glycerol is a good formulation when aiming to produce SPG edible films for packaging applications. Realistic expectations that are made attainable henceforth as a result of this research can be that SPG edible films carry favorable qualities in terms of mechanical qualities, oxygen permeability and water vapor permeability. These properties in SPG films are comparable to the qualities of other polysaccharide-based edible films such as Alyssum homolocarpum, gum ghatti and agar. This research clearly demonstrated that the packaging of food products can be aided by edible SPG films.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbasi S. Challenges towards characterization and applications of a novel hydrocolloid: Persian gum. Curr Opin Colloid Interface Sci. 2017;28:37–45. doi: 10.1016/j.cocis.2017.03.001. [DOI] [Google Scholar]
- Abbasi S. Persian gum: a novel natural hydrocolloid. Nutr Food Sci Res. 2017;4:1–2. [Google Scholar]
- Arham R, Mulyati MT, Metusalach M, Salengke S. Physical and mechanical properties of agar based edible film with glycerol plasticizer. Int Food Res J. 2016;23:1669–1675. [Google Scholar]
- ASTM (1995) Standard test methods for water vapour transmission of materials. In standards designations: E96–95. Annual book of ASTM standards. Philadelphia, Pa: American society for testing and materials.
- ASTM (2001) Standard test method for tensile properties of thin plastic sheeting. Standard D882 ASTM, Annual book of ASTM, pp 162–170.
- Beristain-Bauza SC, Mani-López E, Palou E, López-Malo A. Antimicrobial activity and physical properties of protein films added with cell free supernatant of Lactobacillus rhamnosus. Food Control. 2016;62:44–51. doi: 10.1016/j.foodcont.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Bourtoom T. Edible films and coatings: characteristics and properties. Int Food Res J. 2008;15:237–248. [Google Scholar]
- Chevalier E, Assezat G, Prochazka F, Oulahal N. Development and characterization of a novel edible extruded sheet based on different casein sources and influence of the glycerol concentration. Food Hydrocoll. 2018;75:182–191. doi: 10.1016/j.foodhyd.2017.08.028. [DOI] [Google Scholar]
- Dabestani M, Kadkhodaee R, Phillips GO, Abbasi S. Persian gum: a comprehensive review on its physicochemical and functional properties. Food Hydrocoll. 2018;78:92–99. doi: 10.1016/j.foodhyd.2017.06.006. [DOI] [Google Scholar]
- Daza LD, Homez-Jara A, Solanilla JF, Váquiro HA. Effects of temperature, starch concentration, and plasticizer concentration on the physical properties of ulluco (Ullucustuberosus Caldas)-based edible films. Int J Biol Macromol. 2018;120:1834–1845. doi: 10.1016/j.ijbiomac.2018.09.211. [DOI] [PubMed] [Google Scholar]
- Dick M, Costa TMH, Gomaa A, Subirade M, Rios ADO, Flôres SH. Edible film production from chia seed mucilage: effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr Polym. 2015;130:198–205. doi: 10.1016/j.carbpol.2015.05.040. [DOI] [PubMed] [Google Scholar]
- Ekrami M, Emam-Djomeh Z. Water vapor permeability, optical and mechanical properties of Salep-based edible film. J Food Process Preserv. 2014;38:1812–1820. doi: 10.1111/jfpp.12152. [DOI] [Google Scholar]
- Farhan A, Hani NM. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017;64:48–58. doi: 10.1016/j.foodhyd.2016.10.034. [DOI] [Google Scholar]
- Ghasemlou M, Aliheidari N, Fahmi R, Shojaee-Aliabadi S, KeshavarzB CMJ, Khaksar R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr Polym. 2013;98:1117–1126. doi: 10.1016/j.carbpol.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Haq MA, Hasnain A, Jafri FA, Akbar MF, Khan A. Characterization of edible gum cordia film: effects of beeswax. LWT - Food Sci Technol. 2016;68:674–680. doi: 10.1016/j.lwt.2016.01.011. [DOI] [Google Scholar]
- Heidari M, Rahemi M, Daneshvar MH. Effects of mechanical, chemical scarification and stratification on seed germination of Prunusscoparia (Spach) and Prunuswebbii (Spach) Vierh. Am Eurasian J Agric Environ Sci. 2008;3:114–117. [Google Scholar]
- Iamareerat B, Singh M, Sadiq MB, Anal AK. Reinforced cassava starch based edible film incorporated with essential oil and sodium bentonite nanoclay as food packaging material. J Food Sci Technol. 2018;55:1953–1959. doi: 10.1007/s13197-018-3100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibar EAA, Ferhunde US. starch-cellulose ether films: microstructure and water resistance. J Food Process Eng. 2017;40:1–8. [Google Scholar]
- Liang T, Wang L. Preparation and characterization of a novel edible film based on Artemisia sphaerocephala Krasch. gum: effects of type and concentration of plasticizers. Food Hydrocoll. 2018;77:502–5018. doi: 10.1016/j.foodhyd.2017.10.028. [DOI] [Google Scholar]
- Liu WG, Yao KD. What causes the unfrozen water in polymers: hydrogen bonds between water and polymer chains? Polymer. 2001;42:3943–3947. doi: 10.1016/S0032-3861(00)00726-6. [DOI] [Google Scholar]
- Mohammadi S, Abbasi S, Scanlon MG. Development of emulsifying property in Persian gum using octenylsuccinic anhydride (OSA) Int J Biol Macromol. 2016;89:396–405. doi: 10.1016/j.ijbiomac.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Nafchi AM, Olfat A, Bagheri M, Nouri L, Karim AA, Ariffin F. Preparation and characterization of a novel edible film based on Alyssum homolocarpum seed gum. J Food Sci Technol. 2017;54:1703–1710. doi: 10.1007/s13197-017-2602-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nur- Hanani ZA, Mc-Namara J, Roos YH, Kerry JP. Effect of plasticizer content on the functional properties of extruded gelatine-based composite films. Food Hydrocoll. 2013;31:264–269. doi: 10.1016/j.foodhyd.2012.10.009. [DOI] [Google Scholar]
- Osés J, Niza S, Ziani K, Maté JI. Potato starch edible films to control oxidative rancidity of polyunsaturated lipids: effects of film composition, thickness and water activity. Int J Food Sci Technol. 2009;44:1360–1366. doi: 10.1111/j.1365-2621.2009.01965.x. [DOI] [Google Scholar]
- Ploypetchara T, Gohtani S. Effect of sugar on starch edible film properties: plasticized effect. J Food Sci Technol. 2018;9:3757–3766. doi: 10.1007/s13197-018-3307-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoufi N, Fang Y, Kadkhodaee R, Phillips GO, Najaf-Najafi M. Changes in turbidity, zeta potential and precipitation yield induced by Persian gum-whey protein isolated interactions during acidification. J Food Process Preserv. 2017;41:1–8. doi: 10.1111/jfpp.12975. [DOI] [Google Scholar]
- Sadeghi-Varkani A, Emam-Djomeh Z, Askari G. Physicochemical and microstructural properties of a novel edible film synthesized from Balangu Seed Mucilage. Int J Biol Macromol. 2018;108:1110–1119. doi: 10.1016/j.ijbiomac.2017.11.029. [DOI] [PubMed] [Google Scholar]
- Singh TP, Chatli MK, Sahoo J. Development of chitosan based edible films: process optimization using response surface methodology. J Food Sci Technol. 2015;52:2530–2543. doi: 10.1007/s13197-014-1318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sothornvit R, Pitak N. Oxygen permeability and mechanical properties of banana films. Food Res Int. 2007;40:365–370. doi: 10.1016/j.foodres.2006.10.010. [DOI] [Google Scholar]
- Soukoulis C, Singh P, Macnaughtan W, Parmenter C, Fisk I. Compositional and physicochemical factors governing the viability of Lactobacillus rhamnosus GG embedded in starch-protein based edible films. Food Hydrocoll. 2016;52:876–878. doi: 10.1016/j.foodhyd.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajik S, Maghsoudlou Y, Khodaiyan F, Jafari SM, Ghasemlou M, Aalami M. Soluble soybean polysaccharide: a new carbohydrate to make a biodegradable film for sustainable green packaging. Carbohydr Polym. 2013;97:817–824. doi: 10.1016/j.carbpol.2013.05.037. [DOI] [PubMed] [Google Scholar]
- Tong DW, Mauer LJ, Wongruong S, Sriburi P, Rachtanapun P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem Cent J. 2011;5:6. doi: 10.1186/1752-153X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas M, Albors A, Chiralt A, González-Martínez C. Water interactions and microstructure of chitosan-methylcellulose composite films as affected by ionic concentration. LWT - Food Sci Technol. 2011;44:2290–2295. doi: 10.1016/j.lwt.2011.02.018. [DOI] [Google Scholar]
- Wang X, Guo C, Hao W, Ullah N, Chen L, Li Z, Feng X. Development and characterization of agar-based edible films reinforced with nano-bacterial cellulose. Int J Biol Macromol. 2018;118:722–730. doi: 10.1016/j.ijbiomac.2018.06.089. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhao Y, Shi Q. Characterization of a novel edible film based on gum ghatti: effect of plasticizer type and concentration. Carbohydr Polym. 2016;153:345–355. doi: 10.1016/j.carbpol.2016.07.082. [DOI] [PubMed] [Google Scholar]