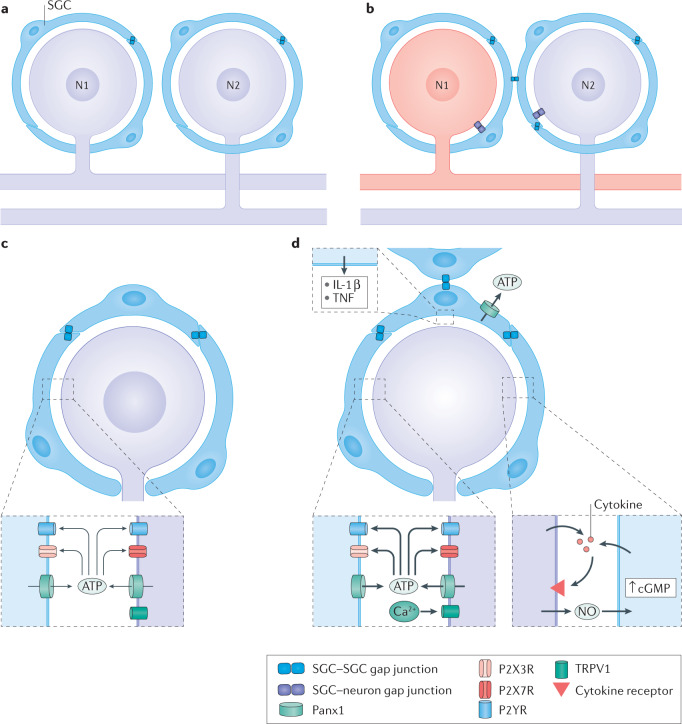
Fig. 3. Injury-induced changes in neuron–SGC bidirectional communications.
a,b | Proposed mechanism of coupled activation between neurons. Synchronous activity of adjacent neurons could arise by the spread of depolarization from neuron 1 (N1) to its surrounding satellite glial cells (SGCs), then through gap junctions to SGCs of a nearby neuron and then through gap junctions from these SGCs to N2. Under control conditions, SGCs are coupled mostly to other SGCs around a given neuron (part a). After peripheral injury or inflammation to a neuron (coloured red), SGCs become more strongly coupled to other SGCs around the same neuron (and also to neurons) by newly formed gap junctions. This enables increased transfer of electrical current and small molecules among SGCs and between SGCs and neurons. Such a mechanism can account for coupled neuronal activation (part b). c,d | The chain of events connecting neuronal excitation and glial activation. Resting conditions are shown in part c. Following neuronal damage, the neuronal cell body fires a high rate of action potentials, which increases intracellular calcium that in turn activates nitric oxide synthase to produce nitric oxide. Nitric oxide diffuses from the neuron and reaches the surrounding SGCs, where it induces cyclic guanosine 5'-monophosphate (cGMP) synthesis. This second messenger can have various actions on SGCs, and may be a key factor in SGC activation. SGC activation includes the release of ATP and cytokines, gap junction formation and increased sensitivity to ATP (part d). NO, nitric oxide; P2R, P2 purinergic receptor; Panx1, pannexin 1; TNF, tumour necrosis factor; TRPV1, transient receptor potential vanilloid type 1 channel.