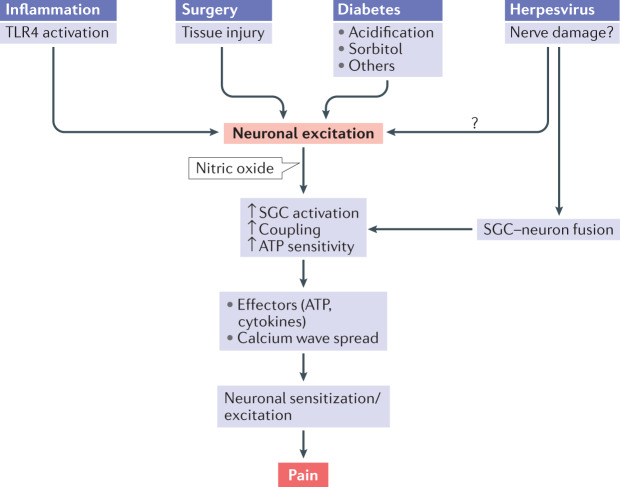
Fig. 4. Proposed sequence of events connecting nerve injury in four different pain models to SGC activation and neuronal hyperexcitability.
This sequence is an attempt to generalize the main events, but does not exclude additional or alternative mechanisms. The initial event in this cascade is injury to sensory neurons, such as from surgery, inflammation, diabetes or herpesvirus, which increases their firing rate. This leads to production of nitric oxide in the neurons. Nitric oxide diffuses from the injured neurons and activates satellite glial cells (SGCs) that surround them and likely also SGCs around adjacent neurons. SGC activation involves numerous processes, including greater coupling by gap junctions, increased sensitivity to ATP, upregulation of ERK and increased release of pro-inflammatory cytokines (IL-1β, tumour necrosis factor, IL-6, fractalkine and others). The increased gap junctions and sensitivity to ATP will enhance calcium waves, which lead to neuronal excitation. Cytokines released from SGCs will also contribute to neuronal sensitization and excitation. The overall effect will be greater firing (spontaneous and/or evoked) of sensory neurons and pain. TLR4, Toll-like receptor 4.