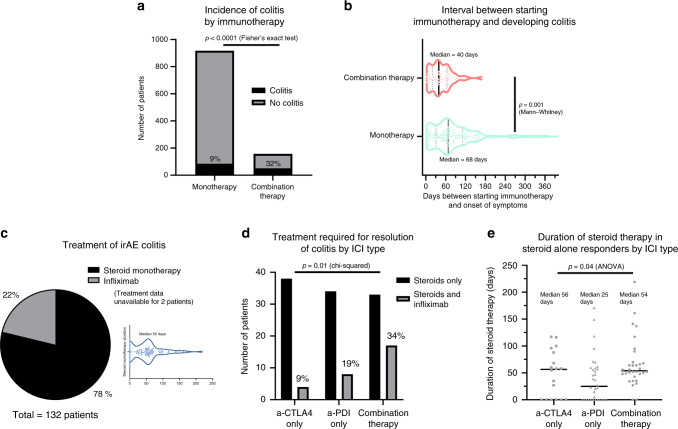
Fig. 1. Presentation and treatment of immunotherapy-induced (irAE) colitis in this cohort (n = 1074) of patients.
a The incidence of colitis in single vs. combination immunotherapy (9% vs. 32%; Fisher’s exact test: p < 0.0001). b Onset of colitis after immunotherapy initiation (median 40 days in combination therapy vs. 68 days in monotherapy; Mann−Whitney test: p = 0.001). c 22% or 29 patients required infliximab for resolution of their colitis. Median duration of steroids in those who were treated with steroids alone was 50 days. d Number of patients requiring steroids monotherapy vs. steroids plus infliximab rescue therapy for treatment of their colitis; subdivided by immunotherapy regimen. Percentages requiring infliximab denoted in figure. Chi-squared test: p = 0.005 for difference between the CTLA4 and the combination therapy groups. e Mean duration of steroids in patients whose colitis responded to steroid monotherapy alone (patients requiring infliximab excluded); subdivided by immunotherapy regimen (median 56 days in anti-CTLA-4, 25 days in anti-PD-1, 54 days in combination; ANOVA: p = 0.04) (N.B. Data unavailable for 50% of patients in the aCTLA4 cohort).