Figure 1.
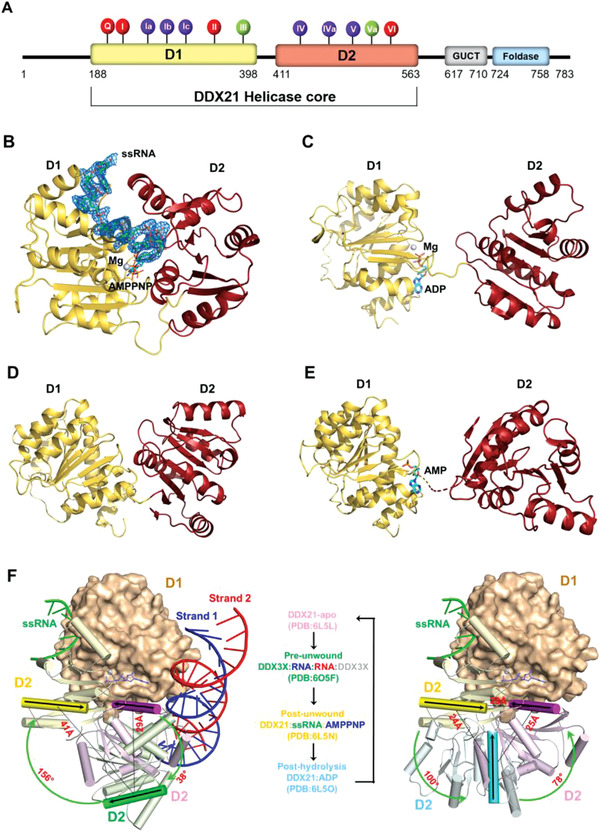
The overall structure of DDX21 helicase core in different unwinding states. A) Domain structure of human DDX21 and conserved sequence motifs of the helicase core. The DDX21 helicase core (D1D2 core, residue 188‐563) was used for structural studies. The 12 highly conserved DDX sequence motifs were colored for their primary functions: red, ATP binding; blue, RNA binding; and green, inter‐domain interaction. See also Figure S1 (Supporting Information). B) Crystal structure of the D1D2 core (yellow/red) bound with ssRNA and AMPPNP‐Mg2+. DDX21 is shown in cartoon model, whereas ssRNA is in a stick model with an electron density map contoured to 3.0 σ at the F o–F c map. C) Crystal structure of the D1D2 core (yellow/red) bound with ADP‐Mg2+. D) Crystal structure of the D1D2 core (yellow/red) in its apo‐state. E) Crystal structure of the D1D2 core (yellow/red) bound with AMP. The disordered part (residues Ile400 – Ile409) connecting the two domains is indicated by a broken line. F) Conformational comparison between the DDX21‐apo (in pink, PDB: 6L5L), DDX3X‐dsRNA (in green, PDB: 6O5F; and only one DDX3X molecule is shown for clarity), DDX21‐ssRNA‐AMPPNP (in yellow, PDB: 6L5N), and DDX21‐ADP (in cyan, PDB: 6L5O) structures. The left panel shows the conformational difference between the apo state, pre‐unwound state and post‐unwound state. The right panel shows the conformational difference between the post‐wound state, post‐hydrolysis and apo state, combining into a four‐step unwinding cycle elucidated in the middle panel. The structures were aligned based on their D1 domains. The D1 of the DDX21‐ssRNA‐AMPPNP structure is illustrated as a molecular surface, and those of other structures are not shown for clarity. The D2 of all four structures are shown as cartoon models (helices as cylinders, strands as arrows, and loops as tubes). The orientation of D2, relative to the fixed D1 at each state, is indicated with a black arrow on the same α10 helix in D2 and a color gradient for emphasis. See also Figure 7 and Figure S3 (Supporting Information).
