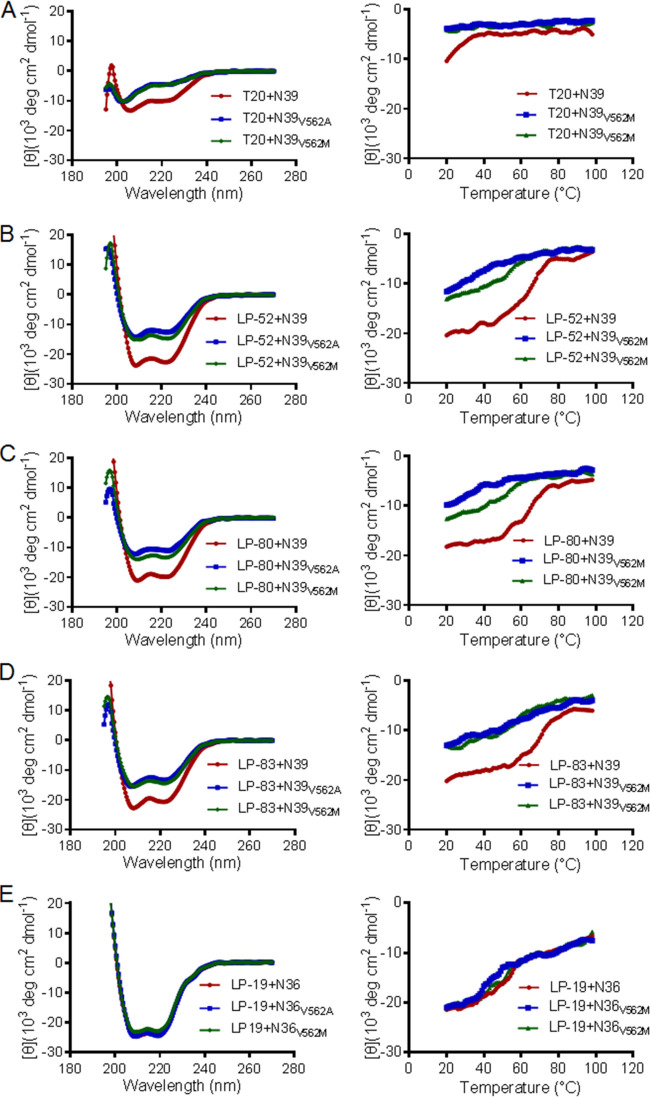
FIG 3.
Effects of the resistance mutations on the binding stability of fusion inhibitors determined by CD spectroscopy. The α-helicity (left panels) and thermostability (right panels) of T20 (A), LP-52 (B), LP-80 (C), LP-83 (D), and LP-19 (E) in complexes with the SIV Env NHR-derived peptide N39 or N36 with a wild-type or mutant sequence were measured with the final concentration of each peptide at 10 μM in PBS. The experiments were performed two times and obtained consistent results, and representative data are shown.