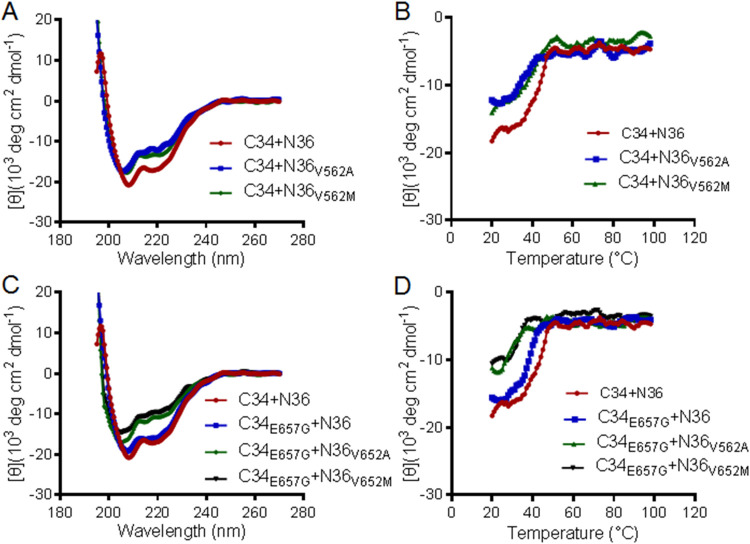
FIG 5.
Effects of the resistance mutations on the helical interaction and stability of 6-HB structure modeled by the NHR and CHR peptides. The α-helicity and thermostability of 6-HBs formed between SIV-derived peptides C34 and N36 or N36 with a V562A or V562M substitution (A and B) and between C34 with an E657G substitution and N36 or its mutants (C and D) were determined by CD spectroscopy. The final concentration of each peptide in PBS was 10 μM. The experiments were performed two times and obtained consistent results, and representative data are shown.