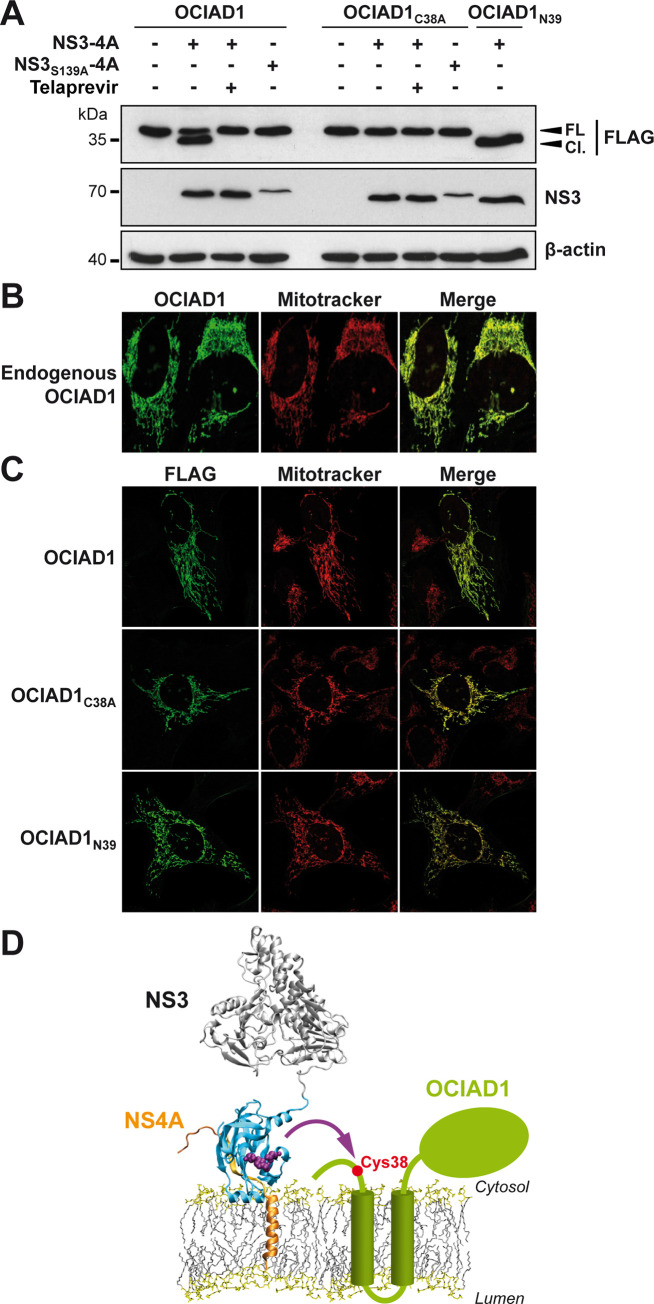
Fig 1. OCIAD1 is a novel cellular substrate of the HCV NS3-4A protease.
(A) U-2 OS cells were transfected with the expression vectors pCMV-OCIAD1-FLAG, pCMV-OCIAD1C38A-FLAG or pCMV-OCIAD1N39-FLAG together with those encoding wild-type or proteolytically inactive (S139A) NS3-4A protease, as indicated. Twelve h post-transfection, cells were treated or not for 24 h with 2.5 μM telaprevir. Cell lysates were separated by 15% SDS-PAGE, followed by immunoblot with monoclonal antibodies anti-FLAG®M2 against the FLAG tag, 1B6 against NS3 or AC-15 against β-actin. Cl., cleaved OCIAD1; FL, full-length OCIAD1. (B) Endogenous OCIAD1 localizes to mitochondria. U-2 OS cells were subjected to immunofluorescence using a polyclonal antibody against OCIAD1 (green) and MitoTracker for staining of mitochondria (red). Mean Pearson’s coefficient was determined as 0.92 (n = 5). (C) NS3-4A–mediated cleavage does not alter the subcellular localization of OCIAD1. U-2 OS cells were transfected with plasmid pCMV-OCIAD1-FLAG (OCIAD1wt), pCMV-OCIAD1C38A-FLAG (OCIAD1C38A), or pCMV-OCIAD1N39-FLAG (OCIAD1N39). Thirty-six h post-transfection, cells were subjected to immunofluorescence using monoclonal antibody anti-FLAG®M2 against the FLAG tag (green) and MitoTracker (red). Mean Pearson’s coefficients were determined as 0.95, 0.85 and 0.88, respectively, for OCIAD1wt, OCIAD1C38A and OCIAD1N39 (n = 5). (D) Tentative model of the cleavage of OCIAD1 by the NS3-4A protease. The model of NS3-4A on the membrane is from Brass V et al. (24). The NS3 serine protease domain is shown in cyan, with the catalytic triad in purple and NS4A in orange. OCIAD1 (green) harbors two predicted transmembrane segments and a C-terminal region oriented toward the cytosolic side. Cys 38 of OCIAD1 is highlighted by a red dot.