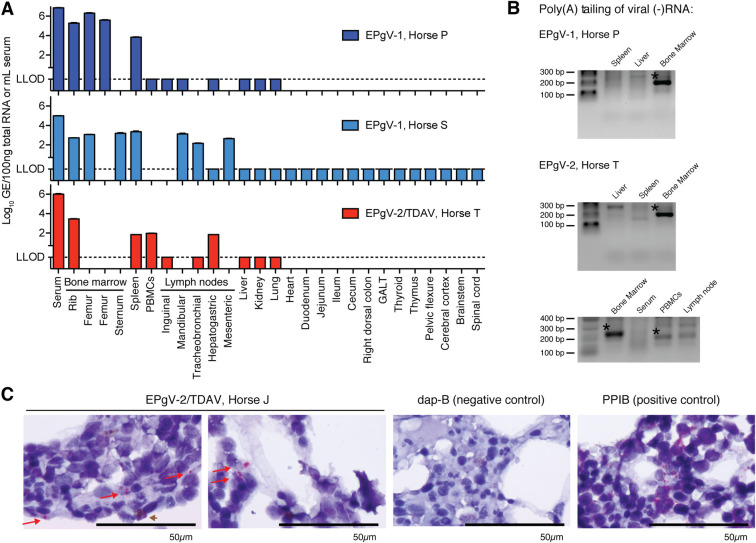
Fig 2. EPgV tissue tropism and site of replication.
(A) Viral RNA levels in serum and tissues. EPgV-1 sampling was done on naturally infected horses positive for at least 8 (Horse P) and 1 (Horse S) months. EPgV-2 sampling was done 29 days post experimental infection with TDAV (except PBMCs on day 28; Horse T). LLOD: lower limit of detection. (B) Negative-strand specific tailing-based RT-PCR performed on bone marrow, spleen and liver for EPgV-1 (Horse P) and bone marrow, spleen, liver, PBMCs and tracheobronchial lymph node for EPgV-2 (Horse T). Expected size of PCR products are 200 and 220 base pairs for EPgV-1 and EPgV-2, respectively. Bands with confirmed viral sequence are indicated with an asterisk. (C) In situ hybridization (ISH) on bone marrow core biopsies for TDAV (Horse J), Dap-B (negative control) and PPIB (positive control) on 1000x magnification images. Images are from sampling on week 5 post inoculation (see lower panel of Fig 4A). Positive pink foci are indicated with red arrows. Note that a reddish-brown pigment (brown arrowhead) in some instances is difficult to distinguish from positive probe labelling. Scale bars represent 50μm.