Figure 2. Prespecified Subgroup Analyses of the Effect of Allopurinol on the Primary Outcome.
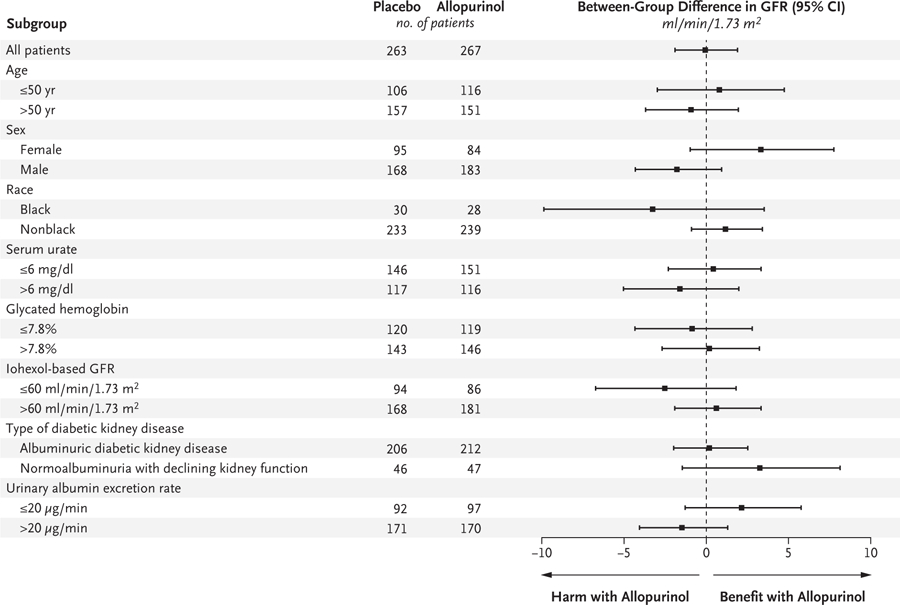
The mean differences in the primary outcome (the iohexol-based GFR at the end of the 2-month washout period) between the allopurinol group and the placebo group are shown in prespecified subgroups. Positive values denote a higher iohexol-based GFR in the allopurinol group than in the placebo group (i.e., benefit with allopurinol); negative values denote a lower iohexol-based GFR in the allopurinol group than in the placebo group (i.e., harm with allopurinol). Race was reported by the patient.
