Fig. 2. Conformational activation and membrane interactions of AP2.
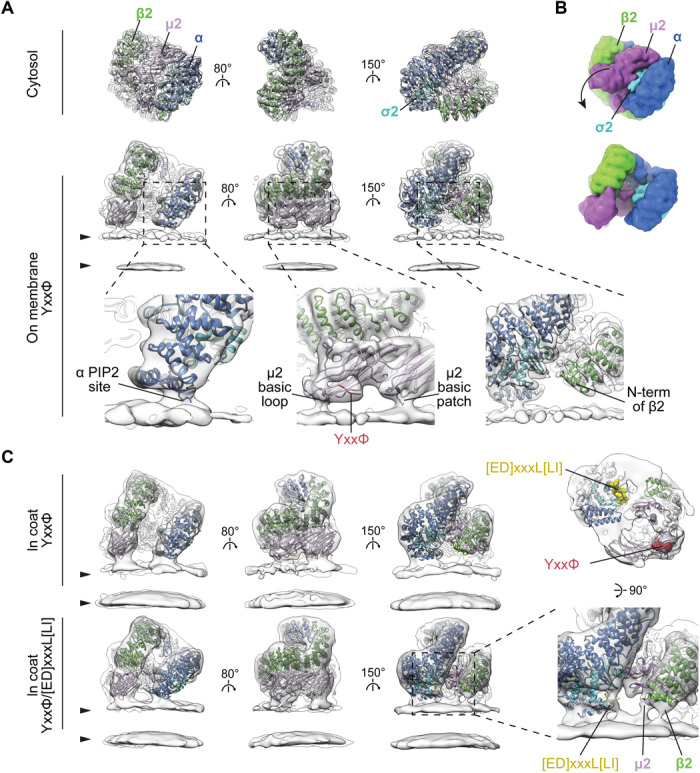
(A) Comparison of single-particle structure of the cytosolic closed form (top) with the tomography-resolved open form of AP2 on the membrane in the presence of YxxΦ cargo (bottom). EM maps (semitransparent gray) are fitted with corresponding ribbon models of AP2 color-coded by chain. Arrowheads indicate membrane leaflets. Close-ups show details of membrane contacts with α and μ2 subunits and the lack of contact with β2, with PtdIns(4,5)P2 sites and cargo peptides marked. The μ2 basic loop includes K167, Y168, R169, and R170, while the μ2 basic patch includes K350, K367, and R368. (B) Surfaces of the atomic models for cytosolic and membrane-recruited AP2 in (A) are shown to illustrate the rearrangement between cytosolic and membrane-bound forms. The arrow indicates the major movement of Cμ2 out of the bowl of AP2. (C) Conformation and membrane interactions of AP2 recruited to the membrane via YxxΦ or YxxΦ/[ED]xxxL[LI] cargo signals within assembled clathrin coats. Additional tilting of α in respect to the membrane is apparent when [ED]xxxL[LI] cargo is present (compare top and bottom panels in the left column). The close-up demonstrates new membrane contacts formed by β2 and μ2 subunits, and additional density in the cargo binding pocket when [ED]xxxL[LI] cargo is present. The panel above the close-up shows YxxΦ/[ED]xxxL[LI]-bound AP2 viewed from the membrane side with peptide-binding sites marked. See also fig. S2E for close-up views of the cargo-binding sites.
