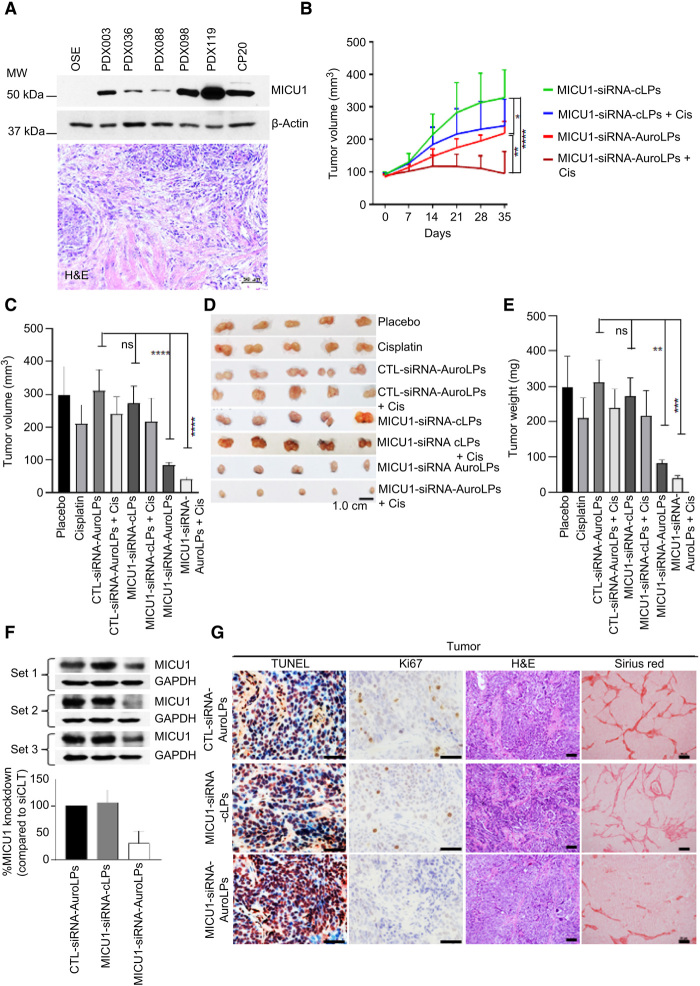
Fig. 4. Antitumor efficacy of MICU1-siRNA-AuroLPs PDX.
(A) MICU1 protein expression in PDX models of primary high-grade serous ovarian cancer. MICU1 expression in different PDX tumors, compared to normal OSE and CP20 cells (top). Histopathological analysis of human ovarian tumor tissues by using H&E to confirm its tumorigenic characteristics (bottom). Scale bar, 50 μm. (B to E) Assessment of antitumor efficacy of auroLPs in PDX mice. PDX-098 was subcutaneously transplanted into NOD/SCID background mice (n = 80). Tumor-bearing PDX model mice (tumor size, 100 mm3) were intravenously injected with cLPs and AuroLPs containing MICU1-siRNA (0.2 mg/kg/thrice weekly) or in combination with intraperitoneal injection of cisplatin (0.5 mg/kg/twice a week). The treatment was continued for 35 days. The tumor volume (tv) was measured weekly (B), and 35-day tv was shown separately (C). Representative tumor images (D) and tumor masses (E) were shown. (F) MICU1 expression in tumor lysates at 35 days with GAPDH as loading control. (G) The representative Ki67-, TUNEL-, H&E-, and Sirius red–stained sections of corresponding tumors. All statistical analyses were performed using one-way ANOVA followed by Dunnett’s multiple comparisons test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, and ****P ≤ 0.0001, n = 5 to 10. Photo credit: M. N. Hossen (OUHSC).