ABSTRACT
Path integration is a straightforward concept with varied connotations that are important to different disciplines concerned with navigation, such as ethology, cognitive science, robotics and neuroscience. In studying the hippocampal formation, it is fruitful to think of path integration as a computation that transforms a sense of motion into a sense of location, continuously integrated with landmark perception. Here, we review experimental evidence that path integration is intimately involved in fundamental properties of place cells and other spatial cells that are thought to support a cognitive abstraction of space in this brain system. We discuss hypotheses about the anatomical and computational origin of path integration in the well-characterized circuits of the rodent limbic system. We highlight how computational frameworks for map-building in robotics and cognitive science alike suggest an essential role for path integration in the creation of a new map in unfamiliar territory, and how this very role can help us make sense of differences in neurophysiological data from novel versus familiar and small versus large environments. Similar computational principles could be at work when the hippocampus builds certain non-spatial representations, such as time intervals or trajectories defined in a sensory stimulus space.
KEY WORDS: Cognitive map, Limbic system, Place cell, Grid cell, Boundary cell, Robot navigation
Summary: Path integration is one of the fundamental computations giving rise to the cognitive map and possibly other non-spatial representations in the hippocampal formation and its subcortical afferents.
Introduction
In essence, path integration is an internal computation that transforms a sense of motion into a sense of location. It requires tracking angular movements and distances traveled to estimate one's current position and orientation relative to a world-based (‘allocentric’) frame of reference. Mathematically, this estimate is a vector in a system of positional and directional coordinates associated with the allocentric reference frame. Position and orientation combined together are termed a ‘pose’ in mobile robotics and sometimes in biology (e.g. Heinze et al., 2018; Thrun et al., 2005) – not to be confused with a more comprehensive account of an animal's posture (e.g. Mimica et al., 2018). If path integration is calculated in a step-wise manner, the new pose update can be obtained by adding a movement vector estimating changes in position and orientation to the last pose update. If not, a continuous pose update can be obtained by integrating in time momentary angular and linear velocities. In either case, the necessary inputs are assumed to be derived from self-motion (‘idiothetic’) cues produced by locomotion (Barlow, 1964; Etienne et al., 1996; Etienne and Jeffery, 2004; Gallistel, 1990; McNaughton et al., 2006). These cues are typically internally generated, such as vestibular (inertial) cues, proprioceptive cues or motor efference copy (e.g. step counting). Hence, path integration is sometimes defined as the processing of these internal cues. However, some idiothetic cues that are not internally generated can be used for path integration, for example, optic flow, airstream detection (e.g. by a rat's whiskers) or other sensory reafference inputs produced by locomotion. Comparable sensors (gyroscopes, odometers, etc.) afford the application of path integration to artificial systems ranging from ships to robots.
To further complicate a comprehensive definition of path integration, the term is sometimes used to refer to components of the general problem of pose estimation. For example, a source of reliable directional information may be available, such as a compass to a sailor or polarized skylight to a desert ant (Fent and Wehner, 1985). In these instances, directional uncertainty is largely removed on a short time scale (on longer ones, compass recalibration or sun movements still need to be dealt with), and path integration only concerns the estimate of position. Vice versa, a process conceptually and computationally equivalent to path integration can be applied to a purely directional reference frame – based on the integration of angular velocity to update orientation irrespective of position – as occurs in the nervous system (Green et al., 2017; Knierim and Zhang, 2012; Skaggs et al., 1995; Taube, 2007; Turner-Evans et al., 2017; Zhang, 1996).
Path integration is typically conceptualized as supporting a navigation strategy that is complementary to ‘allothetic’ navigation. Allothetic navigation makes direct use of distinctive and stable environmental features and of the allocentric spatial relationships they specify. Where perceptual access to these landmarks is interrupted, path integration can be used for continued self-localization. But the pose estimate obtained by path integration in these conditions is expected to drift as a result of sensory and computational noise accruing over time, and therefore allothetic cues are required to prevent or correct this cumulative error.
Because allothetic and idiothetic navigation are often imagined as alternating behavioral strategies depending on the momentary availability of particular spatial inputs, path integration is often considered in isolation from landmark processing. This view originates in at least two contexts. In maritime navigation, self-localization in the open sea, where charted features are out of sight, represented an inescapable and critical problem for centuries until the advent of contemporary technology such as GPS. This problem was addressed via formal path integration calculations carried over a map by a ship officer. These calculations were referred to as ‘dead reckoning’ and must have contributed in no small measure to the formulation of the concept of path integration as was later used in biology (Gallistel, 1990). To demonstrate that animals are capable of path integration/dead reckoning via behavioral analysis, the experimenter must subject an animal to a (temporary) removal of allothetic cues or choose an animal that evolved to pay limited attention to these cues owing to exceptional ecological constraints (such as the desert ant; Wehner and Srinivasan, 1981). These experimental settings may reinforce the operational views of path integration as a standalone computation and of allothetic versus idiothetic navigation as functionally segregated strategies.
List of abbreviations.
- BVC
boundary vector cell
- HD
head direction
- MEC
medial entorhinal cortex
- MS
medial septum
- SLAM
simultaneous localization and mapping
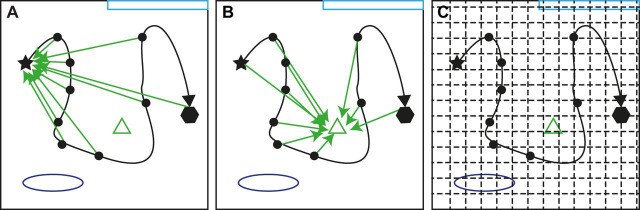
A separate issue is that the behavior called ‘homing’ and the computation called ‘path integration’ are often conflated in the animal behavior literature. Homing refers to an organism's navigation to a home base, often the starting point of a journey. It is the most common behavior used to study path integration, because it is ethologically natural, requires little training, is easy to measure and can be performed in the absence of allothetic landmarks. Because of this prevalence, path integration is often referred to as the process of updating a homing vector. However, homing and path integration are distinct concepts. The behavior of homing can be solved with navigation strategies other than path integration (e.g. a beacon that identifies the home base or starting point). Conversely, the computation of path integration can be used to continuously update a homing vector (Fig. 1A), but it can also be used to update a vector representation to any other fixed point in the environment (Fig. 1B) or to update a position coordinate on a map (Fig. 1C).
Fig. 1.
Possible neural representations of position based on path integration. (A) A homing vector. (B) A vector to a landmark in the environment. (C) A position in a Cartesian coordinate system. Each figure represents an organism's trajectory (black line) from a starting point (black star) to an end point (black hexagon) through an environment that contains landmarks (colored shapes).
Here, we adopt a view of path integration as a continuous pose update that works in tandem, as opposed to alternately, with landmark processing – relative to an arbitrary (but stable) metrical coordinate system (the scenario in Fig. 1C). This view is particularly relevant to the hippocampal cognitive mapping system of the mammalian brain (Etienne and Jeffery, 2004; McNaughton et al., 1996; O'Keefe, 1976; Redish and Touretzky, 1997), where the neural computations underlying path integration and landmark processing are likely to be highly integrated.
Path integration is implicated in fundamental properties of place cells and their interpretation as a cognitive map
Place cells and other spatial cells found in the hippocampal formation signal the position of the animal with respect to an allocentric frame of reference, like the ‘you-are-here’ tag found on a map. After O'Keefe and Dostrovsky's (1971) initial report on place cells, early experimental efforts were dedicated to demonstrating that place cell activity reflected self-localization relative to a cognitive abstraction of allocentric space – the ‘cognitive map’ – and not merely a collection of landmark views or local stimuli that vary systematically with the animal's position (Knierim and Hamilton, 2011). These studies confirmed that place cells can continue to track location when the allothetic spatial cues were removed (Gothard et al., 2001; Quirk et al., 1990; Save et al., 1998), thus implicating idiothetic cues and path integration in the expression of the place cells' firing fields. One of the earliest and most insightful investigations was that of O'Keefe and Speakman (1987), who showed that place fields on a plus maze were controlled by an array of distal landmarks when this array was rotated relative to the maze. However, the place fields could fire in the correct locations on the maze even when the landmarks were removed, as long as the rat was allowed a brief perceptual ‘registration’ period beforehand. Although the rat was confined to a small area of the apparatus during the registration period, place fields were later correctly recalled even when the rat occupied locations outside of this area. These observations revealed a persistent encoding of allocentric self-location and a mechanism capable of extrapolating the registration experience to the remainder of a previously learned map (see also Quirk et al., 1990). Save and colleagues (1998) subsequently showed that blind rats can produce normal place fields in an open arena in which the only salient orientation cues were a peripheral configuration of three objects. Remarkably, reproducible place fields were created even away from these objects. As with sighted rats in a similar apparatus (Cressant et al., 1997), the place fields followed rotation of the object configuration – including place fields located far away from the objects – after the animal had the opportunity to contact the objects. These observations revealed the ability of the place cell representation to persist when the familiar landmarks were not readily available, as well as to interpolate positions ranging between these landmarks.
Although other experiments have corroborated the role of idiothetic cues and the fundamental role played by path integration in hippocampal representations (e.g. Gothard et al., 1996; Terrazas et al., 2005; Bjerknes et al., 2018; Arleo et al., 2013; Knierim et al., 1995, 1998; Sharp et al., 1995; Knierim, 2002; see Moser et al., 2017 for a comprehensive review), direct investigations have proved more challenging than those addressing allothetic cues and landmark processing. Classic laboratory tasks do not lend themselves to the continuous manipulation of idiothetic cues as required by the analysis of path integration, and they are typically limited in space, making it difficult to reveal path integration errors presumably occurring at more ethologically relevant spatial scales. The advent of virtual reality systems has helped overcome some of these limitations (Harvey et al., 2009; Chen et al., 2013; Ravassard et al., 2013; Jayakumar et al., 2019). The discovery of grid cells (Hafting et al., 2005) has further provided new opportunities to make inroads into the neural basis of path integration in the cognitive map.
Grid cells probably reflect path integration and its interaction with allothetic information
Since their discovery in the medial entorhinal cortex (MEC) (Hafting et al., 2005) and the adjacent para- and pre-subiculum areas (Boccara et al., 2010), ‘grid cells’ have offered a fruitful clue into the neural substrate of the metrics of the cognitive map. A single grid cell fires at regular intervals in space, yielding a hexagonal grid of place fields (or, more precisely, a regular grid of equilateral triangles) that spans the full navigation range available to the animal (Figs 2B and 3A). Grid patterns of different cells vary in scale, orientation and phase (Barry et al., 2007; Stensola et al., 2012), and they can maintain consistent geometric relationships to each other across environments or experimental manipulations (Fyhn et al., 2007; Savelli et al., 2017; Yoon et al., 2013).
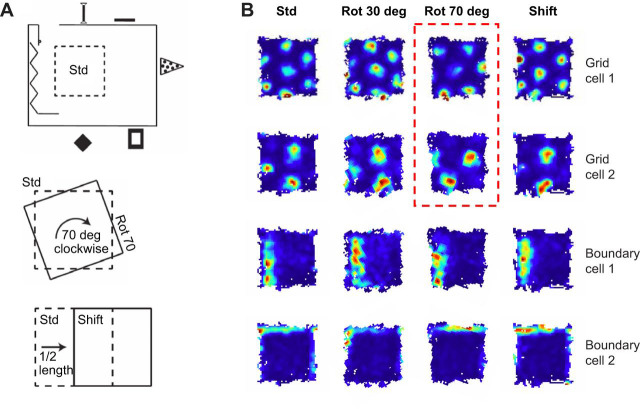
Fig. 2.
Grid and boundary cells in affine transformations (I). (A) Schematic of an experimental apparatus in which rats foraged on a 1.4×1.4 m platform [standard (Std)] placed in a room enriched with remote visual cues (top). This platform was rotated (Rot)/shifted (Shift) between recording sessions (center and bottom). (B) Example of two grid cells of different scale and two boundary cells that were recorded simultaneously across consecutive manipulations. The color code in each rate map represents the average firing rate of the cell in each position on the platform (red: high firing rate; blue: lack of activity). The rate maps have been rotated to a common orientation so as to aid visual comparison. The geometric relationships between each grid pattern and the boundaries (as well as the firing fields of the boundary cells) remain constant across most manipulations, consistent with the idea that boundary cells can predictably anchor the grid representation to an allocentric reference frame. However, in some cases, these relationships can be reconfigured because the grids drifted relative to the platform reference frame (marked by dashed red lines) while controlled by distal cues (not illustrated). Adapted from Savelli et al. (2017).
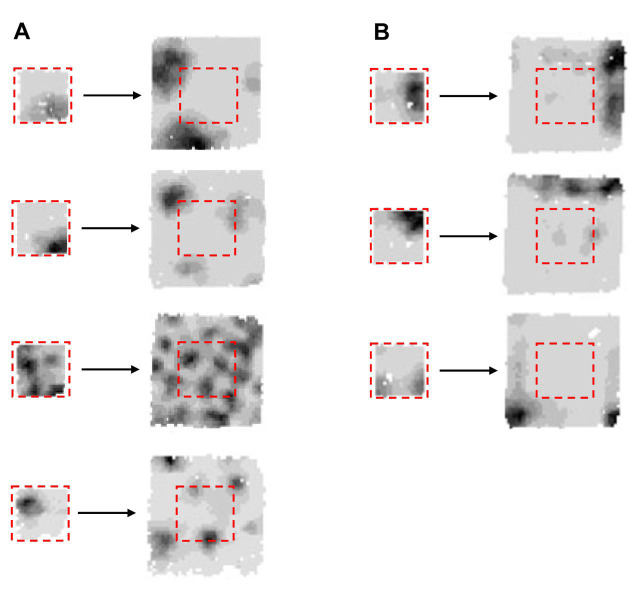
Fig. 3.
Grid and boundary cells in affine transformations (II). Rate maps of grid cells (A) and boundary cells (B) recorded first in a small box (58×58 cm) and then in a larger box (135×135 cm) in a single, uninterrupted session (these cells were not simultaneously recorded). Darker shades indicate a higher level of firing. Dashed red line indicates the prior position of the small box in the large box. The larger box revealed the qualitatively different firing patterns of these two types of cells, but in the small box it is usually impossible to tell one type from the other just based on the appearance of their firing fields (compare, for example, cell in A versus cell in B on each row). The specific influence of grid cells on downstream place cells is thus likely to become greater in larger environments where the rat can travel far from the boundaries encoded by boundary cells. Data from Savelli et al. (2008).
Every grid cell develops firing locations in any environment (Fyhn et al., 2007; Hafting et al., 2005). It is hard to imagine how the geometric arrangement of these firing locations could be directly imparted by a matching arrangement of environmental stimuli, in every environment and for all the different coexisting grids. Rather, an internal process seems at play, which geometrically interrelates environment locations that are experienced serially, based on a metric inherent in the animal's movements. Path integration is an obvious candidate for this process, as it can be used to keep track of the distance between grid firing positions relative to a single coordinate frame (Fig. 1C). Intermixed anatomically with grid cells are neural correlates of speed, direction and conjunctive location×direction (Cacucci et al., 2004; Sargolini et al., 2006; Kropff et al., 2015) that conjure the tell-tale computational signatures of a path integrator at work in the hippocampal formation (discussed below). Moreover, genetic manipulations of entorhinal cells support a causal role of these cells in the behavioral performance of tasks requiring path integration (Gil et al., 2018; Tennant et al., 2018; but see Bjerknes et al., 2018 for evidence that place cells can reflect path integration in darkness before well-formed grid cells develop postnatally). Accordingly, theoretical models implicate grid cells in path planning functions (Banino et al., 2018; Bush et al., 2015; Erdem and Hasselmo, 2012, 2014; Kubie and Fenton, 2012; Stemmler et al., 2015), and several computational models of grid signal generation are based on neural models of path integration (discussed below). Grid-like firing has also been shown to emerge in general-purpose machine-learning systems trained to perform path integration (Banino et al., 2018; Cueva and Wei, 2018 preprint). [Models of grid cells that are not formally based on path integration have nonetheless been proposed (Franzius et al., 2007; Kropff and Treves, 2008; Si and Treves, 2013; Urdapilleta et al., 2017; Weber and Sprekeler, 2018).]
However, in spite of path integration being an inherently noisy computation, the firing patterns of grid cells remain spatially stable over prolonged and repeated trials, suggesting that landmarks provide a spatial reference for path integration. In other words, while the grid spatial code seems to reflect the animal's ability to egocentrically parse the metrical structure of the external world by path integration, the resulting neural representation is nonetheless allocentric. Prominent landmarks that exert strong influence over spatial behavior are the geometric boundaries of the environment (Cheng, 2008, 1986; Cheng and Newcombe, 2005; Tommasi et al., 2012). Based on the responses of place cells to boundary manipulations, Burgess, O'Keefe and colleagues proposed the existence of ‘boundary vector cells’ (BVCs) (Barry et al., 2006; Hartley et al., 2000; O'Keefe and Burgess, 1996). A BVC is postulated to fire whenever the animal is at a certain distance from any boundary in a certain allocentric direction. Subsequent experimental studies discovered cells that encoded the animal's position relative to boundaries – the walls or edges constituting the perimeter of the recording arena (‘boundary’ or ‘border’ cells) (Lever et al., 2009; Savelli et al., 2008; Solstad et al., 2008) (Figs 2B and 3B). Boundary cells are intermixed with grid cells in all brain areas where the latter have been also found (with perhaps the exception of the subiculum, which does not seem to contain grid cells, but see Brotons-Mas et al., 2017; Sharp, 1999). Do boundary cells functionally complement path integration by endowing the grid representation with allocentric properties? Recent analyses have shown that the positional signal encoded by grid cells tends to drift in open space until the animal encounters a boundary, where the drift is corrected by ‘resetting’ the encoded position (Hardcastle et al., 2015). Because a drift is indeed expected to occur because of accumulating errors in path integration, this finding is consistent with the idea that synaptic inputs from boundary cells episodically reset a grid cell signal that is produced continuously through path integration (Barry et al., 2007; Burgess et al., 2007; Cheung et al., 2012; Fuhs and Touretzky, 2006; Hasselmo, 2008; Knierim and Hamilton, 2011; McNaughton et al., 1996; Redish and Touretzky, 1997; Savelli et al., 2008; Solstad et al., 2008; Zhang et al., 2014). The consistency of these corrections across boundaries guarantees that this signal is transformed into a proper allocentric representation.
There are, however, two caveats concerning the relationship between grid cells, boundary cells and path integration. First, grid firing patterns can exhibit elliptical compression/stretching, shearing or other types of distortion (Barry et al., 2007; Krupic et al., 2015; Savelli et al., 2017, 2008; Stensola et al., 2012, 2015). Some of these distortions can develop in time-averaged data plots if different boundaries reset the grid in a slightly inconsistent way relative to a single reference frame (Keinath et al., 2018). If so, the distortions observed in these plots result, to some degree, from the allocentric ‘jittering’ of the coordinate system produced by encountering specific boundaries, rather than from metrical errors of the path integrator. An alternative interpretation of the grid distortions is that the grid representation is not a universal metric for the cognitive map; rather, it purposely reflects the geometric peculiarities of the environment (Krupic et al., 2016). Resolving the function and origin of grid distortions is a crucial future direction in grid cell research as it promises to reveal more of their function and neural mechanisms, especially as to their relation with path integration and with path integration's computational limits in the rat.
A second caveat concerns the likely existence of other strategies for stabilizing the grid representation besides the use of boundaries. The grid representation can be distally controlled by remote landmarks beyond the navigation range experienced by the animal, causing grids to geometrically dissociate from both the physical local boundaries and their neural representation via boundary cells (Savelli et al., 2017) (Fig. 2B). This scenario implies a plastic relationship between grid and boundary cells, which may be reconfigured by BVCs anchoring the grid representation to the remote laboratory walls, or by landmark vector cells (Deshmukh and Knierim, 2013, 2011; Hoydal et al., 2018 preprint; McNaughton et al., 1995; Wilber et al., 2014) doing the same relative to discrete remote landmarks (or by a yet-undiscovered representation of the geometrical center of the environment; see discussion in Savelli et al., 2017).
The ubiquitous, ‘universal’ manifestation of grid cells' periodic firing patterns in any environment evokes the image of a reusable stock of graph paper on which cognitive maps could be charted at multiple spatial resolutions. Does the grid cell system set the metrics and reference frame for the whole cognitive map across the hippocampal formation (Moser and Moser, 2008)? A recent study shows that place-cell maps inherit at least some of the grid-map geometrical properties, as knockout of HCN1 channels in the MEC increased the scale of the grids in the MEC as well as place field size in the downstream hippocampus (Mallory et al., 2018). The influence of grid inputs on the place-cell map is additionally supported by experimental manipulations of entorhinal cells leading to place cell ‘remapping’ (Fyhn et al., 2007; Kanter et al., 2017; Miao et al., 2015; Rueckemann et al., 2016).
Where, and how, does path integration occur?
There seem to be at least two main scenarios to consider for the anatomical and computational origin of path integration contributions to hippocampal cognitive maps.
Cortical attractors
In the first scenario, path integration is computed in the cerebral cortex, in the MEC and possibly in other cortical regions of the hippocampal formation. Putative path integration inputs are present in the MEC in the form of velocity signals. Speed is encoded in multiple ways (Hinman et al., 2016) by speed cells (Kropff et al., 2015), by speed-modulated firing rates of spatial cells (Sargolini et al., 2006) or by the exact frequency of theta-modulated bursts of these cells (Jeewajee et al., 2008). Orientation can be provided by head direction (HD) cells (Sargolini et al., 2006), which fire selectively when the animal's head is pointed in a particular allocentric direction (Taube, 2007) (but see Raudies et al., 2015 for a discussion about a critical difference between head and moving direction). Putative path integration outputs – or its intermediate computational results – are also present in the MEC in the form of allocentric positional and directional representations encoded by grid cells (Hafting et al., 2005), grid×HD cells (Sargolini et al., 2006) and possibly other non-periodic spatial cells (Diehl et al., 2017; Hardcastle et al., 2017; Keene et al., 2016). This collection of experimental phenomena supports models of grid-pattern generation by path-integration-performing ‘continuous attractor’ networks (Knierim and Zhang, 2012; McNaughton et al., 2006). In this class of models, a persistent pattern of neural activity (the ‘activity bump’) propagates through the network in lockstep with the animal's locomotion, guided by velocity signals and mediated by recurrent connections. If the network is topologically arranged as a torus, any cell fires every time the bump returns to the same cell, possibly after completing an arbitrary loop around the torus, thus giving rise to a rectangular grid of firing fields repeating in space (McNaughton et al., 2006). If the neural connections on the torus are systematically twisted, this grid becomes rhomboidal (instead of rectangular), similar to that expressed by a real grid cell (Guanella et al., 2007). Alternatively, multiple bumps are continuously generated and move in concert in the network. Repeating fields in this case result from different bumps riding over the same cell. The hexagonal pattern of bumps that would make the cell fire as a real grid cell can spontaneously emerge in such a network if lateral excitation and inhibition are fashioned as a Turing reaction–diffusion system (Burak and Fiete, 2009; Fuhs and Touretzky, 2006; McNaughton et al., 2006). More recently, the recurrent connections between putative grid cells were primarily found to involve inhibitory interneurons, and attractor models have been successfully refined to accommodate this finding (Couey et al., 2013; Pastoll et al., 2013; Shipston-Sharman et al., 2016).
Subcortical oscillators
In the second scenario, only allothetic information is processed cortically, whereas path integration computations are carried out subcortically, similar to the anatomical and computational organization of the HD cell system (Blair et al., 2008). Like place and grid cells, HD cells also depend on the consistent processing of both idiothetic and allothetic cues, albeit restricted to a purely angular reference frame. The HD signal originates from subcortical regions processing angular velocity signals, presumably based on motor and vestibular inputs (Cullen and Taube, 2017; Taube, 2007). This signal then reaches the cortical postsubiculum, where it is aligned with visual landmarks (Goodridge and Taube, 1997). Last, the landmark-aligned signal is fed back to the same subcortical areas where the HD signal originated (Yoder et al., 2015). Thus, the HD representation is initiated in subcortical circuits performing angular path integration, but its allocentric character is acquired in a cortical area and propagated back to the originating circuits, to ensure the coherence of the internal sense of direction throughout the brain.
The cortical versus subcortical contributions to the place/grid cell system may be similarly organized. First, the positional signals of this system could be initiated via path integration in the medial septum (MS) or upstream of it. The MS contains theta cells, which are interneurons modulated by the 6–12 Hz theta rhythm that dominates hippocampal activity during locomotion (see Buzsáki, 2002 for a review). The bursting frequency of these cells is modulated by speed and direction of locomotion (King et al., 1998; Welday et al., 2011), providing a representation of velocity based on a temporal code. They have been proposed to additionally encode the position of the animal via their relative theta firing phase – a ‘phase code’ (Blair et al., 2008; Monaco et al., 2011; Welday et al., 2011) – building on previous theoretical work on ‘velocity-modulated oscillatory interference models’ (Burgess et al., 2007; Hasselmo et al., 2007). The proposed neural architecture comprises subcortical banks of velocity-modulated ‘ring attractors’ (Blair et al., 2014, 2008; Burgess and Burgess, 2014), in which a bump of activity is hypothesized to periodically traverse a network that is topologically arranged as a ring. Each ring works as a path integrator that tracks the distance traveled in a particular direction by the velocity-modulated precession of its phase relative to a reference theta oscillation. Ring attractors had previously been hypothesized to explain angular path integration in HD cells (Cullen and Taube, 2017; Green et al., 2017; Kim et al., 2017; Knierim and Zhang, 2012; Turner-Evans et al., 2017). Thus, path integration in two-dimensional space may have evolved by duplicating and repurposing pre-existing subcortical circuitry originally dedicated to a one-dimensional version of ‘path integration’ in angular space for HD cells (Blair et al., 2008). Besides the work of Welday et al. (2011), recent studies have implicated the MS in the processing or relaying of idiothetic information (Fuhrmann et al., 2015; Hinman et al., 2016; Justus et al., 2017).
Second, the positional estimate represented by the distribution of phases across the subcortical bank of velocity-controlled ring attractors may be projected to cortical areas such as the MEC (Gonzalez-Sulser et al., 2014; Justus et al., 2017). Here, this signal would be allocentrically reconciled with the representations of boundaries or other landmarks. Grid cells could be a neural product of this process, whereby the subcortical phase-coded output is converted to a rate code that is presumably more amenable to the type of cortical plasticity necessary to bind it to landmark representations (Blair et al., 2014, 2008; Burgess and Burgess, 2014; Burgess et al., 2007; Evans et al., 2016; Hasselmo et al., 2007; Hasselmo and Brandon, 2012). MS pharmacological inactivation severely disrupts the firing patterns of grid cells, although they can still display multiple place fields (Koenig et al., 2011) and preserve any directional modulation (Brandon et al., 2011), suggesting that the disruption is specific to the processing or relaying of metrical information to the grid cell system. Accordingly, rats' ability to estimate linear distances is impaired after MS lesions (Jacob et al., 2017), and the firing locations of place cells shift toward landmarks during MS inactivation (Fattahi et al., 2018).
Last, the allocentric stabilization produced in these cortical areas – or as further processed by the hippocampus proper – would be relayed back to the subcortical representations, making them allocentric as well. Indeed, neurons of the lateral septum signal allocentric self-position by both firing rate and theta phase (Leutgeb and Mizumori, 2002; Takamura et al., 2006; Tingley and Buzsáki, 2018; Monaco et al., 2019), while neurons in other subcortical areas also encode position by firing rate (Jankowski et al., 2015; Jankowski and O'Mara, 2015). [Similarly, speed is encoded both by rate and phase in the MEC, but with a different degree of sensitivity to MS lesions (Hinman et al., 2016).]
What is path integration good for in the mammalian brain?
In all the scenarios considered above, path integration is viewed as a continuously operating, automatic process closely integrated with landmark processing. Although this computational strategy has long been central to certain theoretical views of hippocampal function (Etienne and Jeffery, 2004; McNaughton et al., 1996; O'Keefe, 1976; Redish and Touretzky, 1997; Taube, 2007), it found practical validation and mathematical formulations in the field of mobile robotics (Thrun et al., 2005). Here, the robot's perceptual and spatial uncertainty is explicitly quantified by probability distributions over a suitable set of random variables modeling: (1) noisy sensory inputs (from both idiothetic sensors, such as odometers and gyroscopes, and allothetic ones, such as cameras, sonar and laser range finders); (2) the effect of locomotor commands (also an idiothetic cue by analogy to motor efference copy); and (3) the robot's pose (i.e. its position and orientation relative to the allocentric reference frame) – all with respect to a given map. Bayesian algorithms perform continuous autonomous localization as a live update of the probability distribution on (3) by integrating the information from (1) and (2) (Fig. 4).
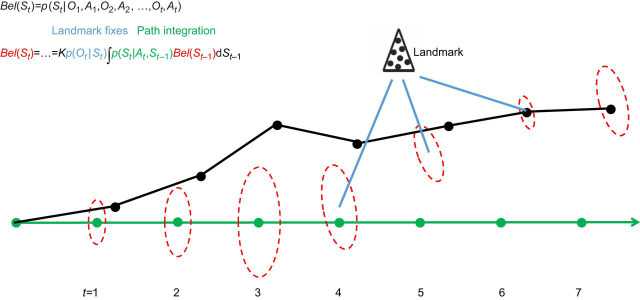
Fig. 4.
Path integration and landmark processing are closely integrated in recursive Bayesian filters for robot self-localization. A ‘belief’ on a state is modeled as a random vector and its multidimensional probability distribution. The cartoon illustrates a self-localization problem where St is a pose, and the associated posterior distribution Bel(St) is conditioned to the history of locomotion actions A1…At and landmark observations O1…Ot, i.e. all that the robot knows. The robot sets on a linear path by sending a series of identical forward motor commands to its wheel actuators (green). The actual path followed by the robot (black) drifts from the intended path. At each step, an update of the self-location estimate is given by recursive equations that calculate Bel(St) from Bel(St–1). The term p(Ot|St) is a conditional distribution on the landmark observations given the current state – it contributes landmark fixes to self-localization. The term p(St|At,St–1) is the conditional distribution on the possible current poses given the action just taken and the previous pose – it contributes path integration to self-localization. The dashed ellipses denote the mean and variance of Bel(St) derived by the filter at each step (heading is not represented in this illustration), i.e. they represent the robot's belief of where it is and its associated degree of confidence. When the landmark is out of sight, p(Ot|St) is not informative, and both spatial error and uncertainty grow. When the landmark becomes visible (steps 4–6), both p(Ot|St) and p(St|At,St–1) meaningfully contribute to the estimate, and the landmark is used to reduce both the error and uncertainty (see Thrun et al., 2005 for more details). Note how path integration and landmark fixes are seamlessly and dynamically integrated in this mathematical framework. The idiothetic cue used for path integration in this example is akin to a biological motor efference copy. Other idiothetic cues can also be used, such as given by a gyroscope (vestibular system), odometer (proprioceptive inputs), etc. Also note that p(Ot|St) contains information on what landmark observations are expected at any pose, i.e. it amounts to a map of the environment given to the robot.
Because of its clear computational abstraction, the Bayesian framework may help refine and advance theoretical thinking on hippocampal spatial function (Cheung, 2016; Erdem et al., 2015; Finkelstein et al., 2016; Kanitscheider and Fiete, 2017; Touretzky et al., 2005). Concerning the role of path integration, one advantage of its continuous integration in the process of self-localization may be a rapid, moment-by-moment updating of the position estimate. Landmark identification and distance estimation relative to an internal map are presumably slower perceptual and cognitive processes than the mathematical integration of acceleration/velocity signals, which can be produced in thalamic or primary sensory areas, and partly during sensory transduction (e.g. with vestibular inputs). A system that activated path integration computations only when landmarks become unavailable would arguably be less reactive to rapid movements compared with a system that continually employed path integration; that is, a ‘quick and dirty’ update from path integration (later corrected by landmarks if needed) may be better than a delayed update directly from landmarks. A further related advantage of path integration for navigation is a more reactive response to the sudden or intermittent occlusion of key landmark inputs, for example, in darkness. The equation in Fig. 4 would simply account for this situation as a temporary lack of allothetic inputs in the corresponding term of the equation. These inputs would therefore be subtracted from the self-localization computations, leaving path integration alone performing this job, rather than path integration being added as a ‘backup’ system – a mechanistic difference that may be difficult to detect in behavioral studies.
The tremendous success of the Bayesian approach to mobile robotics, however, is best proven in situations where the robot is not given a reliable map but needs to build it from scratch in the process of exploring a novel environment. In this situation – called concurrent mapping and localization (CML) or simultaneous localization and mapping (SLAM) (Thrun et al., 2005) – the robot faces a chicken-or-egg problem: it needs a map with respect to which it can perform self-localization, but it needs accurate self-localization to stitch together its perceptual views into a map. Therefore, the map itself is subject to uncertainty and it too must be represented by a complex set of multidimensional random variables and their associated probability distributions. SLAM can be solved by a recursive Bayesian update of the joint distribution on the pose and map, thus embracing the circular nature of this problem, but at the cost of adding considerable computational complexity to its algorithmic solution (Thrun et al., 2005) (Fig. 5).
Fig. 5.
Path integration and landmark processing in robot simultaneous localization and mapping (SLAM). In this case, a distribution on the observations of landmarks such as p(Ot|St), described in Fig. 4, is not given to the robot. Instead, a map needs to be built during the exploration of the environment. A circular problem arises in which a map is needed for self-localization, but accurate self-localization relative to this map is needed to extend it to new territory. In this example, the map is a collection of unknown allocentric coordinates for consecutively encountered landmarks. This information can be modeled as a random vector M, so that the goal of the robot is to estimate its joint belief on both the map and its pose relative to it: p(St ,M|O1,A1,O2,A2,…,Ot ,At) (compare with target belief distribution in Fig. 4, in which M does not appear). This problem can still be addressed by recurrent Bayes filters similar in principle to those described in Fig. 4, but with greater mathematical complexity and computational cost. (A) The robot's path is depicted in black, the distribution on its pose at each step is depicted by the fine-dashed ellipses (red) as in Fig. 4, and the distributions on the allocentric landmark poses (contained in M) are similarly depicted as coarse-dashed ellipses (blue). Note how the spatial error and uncertainty progressively grow along the trajectory as in Fig. 4 for both the robot and the landmark poses – unlike in Fig. 4, the landmarks are not useful for self-localization the first time they are encountered. (B) However, when the robot comes back to a position where the first landmark is recognized, the Bayesian algorithm is able to reduce at once the error and uncertainty on its current pose (small red ellipse) and all the landmark poses (shrunken blue ellipses), because the relationships between robot and landmark poses had been embedded along the way within a single coordinate system. These relationships, however erroneous at first, are initially afforded by path integration. Further excursions beyond the initial trajectory can iteratively build up a representation of an extended environment. Other types of map representations, and various mathematical formulations and algorithmic strategies for Bayesian solutions to SLAM, are reviewed in Thrun et al. (2005).
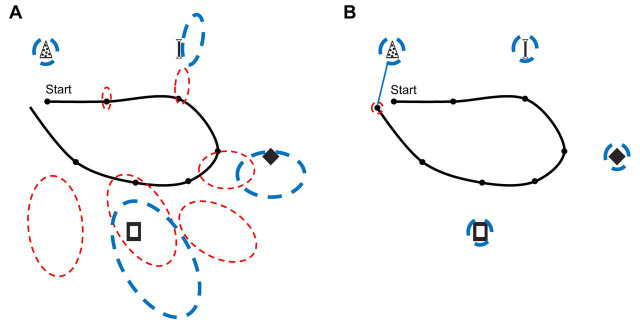
The intuitions derived by this engineering framework converge with theoretical insights from ethological, behavioral and cognitive studies (Fig. 6A) (Gallistel, 1990), in which some form of path integration is deemed necessary for integrating egocentric percepts into a consistent allocentric representation (Alexander and Nitz, 2017, 2015; Byrne et al., 2007; Holmes et al., 2018; Nitz, 2009, 2006; Peyrache et al., 2017; Whitlock et al., 2012; Wilber et al., 2014; Wang et al., 2018). When the map of a new environment is first learned, landmarks are of no immediate help for correcting path integration drift if they are not first charted relative to the allocentric reference frame in use, a task requiring path integration to begin with (Fig. 5). Consideration of how animals explore novel environments is informative here (see also review by Thompson et al., 2018). In a new environment, animals quickly adopt a ‘home base’, often near a salient landmark (Hines and Whishaw, 2005; Wallace et al., 2002), from which they initiate bouts of exploratory excursions and to which they frequently return (Eilam and Golani, 1989). Initial excursions are short, characterized by forward movement interspersed with intermittent pauses (Benjamini et al., 2011; Golani et al., 1993; Kramer and McLaughlin, 2001). During these pauses, animals engage in a behavior called ‘head scanning’, in which they perform lateral head movements to gather information about landmarks and other sensory information available at that location. This behavior has been associated with the rapid, one-shot formation of new place fields at the site of the scan (Monaco et al., 2014; see also Bittner et al., 2017; Diamantaki et al., 2018; Dragoi et al., 2003; Frank et al., 2004). Successive excursions originating from and returning to the home base (interleaving forward motion with head scanning epochs) cover ever-increasing areas of the environment. It is likely that that this pattern of behavior reflects nature's solution to the SLAM problem. During the initial, brief excursion, the animal updates its position based on path integration, providing a spatial framework upon which landmarks are incorporated during head scans. Before excessive path integration error can accumulate, the animal returns to its home base, where any small error can be corrected and the path integrator coordinate system can be reset. Because of the incorporation of landmarks in the initial excursion, the animal can explore a larger area of terrain on its next excursion, as the landmarks prevent error accumulation during the portion of the excursion that repeats the first excursion. Thus, with this iterative process, in which path integration sets the metric of a spatial framework upon which allothetic cues are bound, the animal is able to construct a map of a novel environment. [It is important to keep in mind that compartmentalized environments and large-scale spaces may not be mapped within a single reference frame. Alternative mapping strategies have been the subject of multidisciplinary research (e.g. Derdikman et al., 2009; Kuipers, 2000; Kuipers et al., 2004; Nitz, 2009; Redish, 1999).]
Fig. 6.
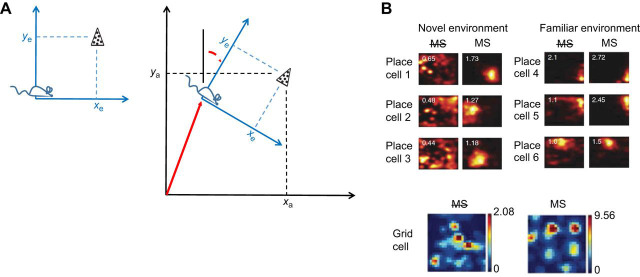
Converging insights into the role of path integration in map building: cognitive models and neurophysiological data are broadly consistent with approaches to robot SLAM. (A) Behavioral studies suggest the existence of map-like cognitive representations in many species. In a novel environment, such a representation needs to be populated by salient environmental features. These features are egocentrically perceived (e.g. coordinates xe,ye of a visual landmark, left plot), but they eventually need to be charted relative to the stable allocentric reference frame adopted for the map (coordinates xa,ya, right plot). This can be achieved mathematically by a coordinate transformation (translation+rotation) based on parameters derived from path integration (red translation vector and rotation angle in red defining the animal's pose in the map frame, right plot). Albeit much simpler, this general model assigns a similar role to path integration in map-building as does the SLAM framework of Fig. 5. [See fig. 5.1 of Gallistel (1990) for more details and analysis extended to algebraic representations of curves and surfaces, reminiscent of the boundaries of an environment.] (B) One implication of this perspective is that the disruption of path integration or its outputs should lead to reduced spatial modulation of the firing fields of place cells in novel environments, but not necessarily in familiar ones, where self-localization can rely on previously charted landmarks (as in Fig. 4). Top: place fields from the same place cells are disrupted in a novel, large enclosure if the medial septum is inactivated (MS with strikethrough), but not when the platform is highly familiar (adapted from Wang et al., 2015). Bottom: when the medial septum is inactivated, grid cells lose their characteristically regular firing pattern, which may be sustained by path integration (adapted from Koenig et al., 2011).
Recent observations appear consistent with the role of path integration in the creation of a new place cell map. As mentioned before, inactivation of the MS suppresses the regular structure in grid cell firing (Brandon et al., 2011; Koenig et al., 2011), consistent with the loss of path integration. In one experiment in a large, novel environment, place cells did not form spatial firing fields during MS inactivation (Wang et al., 2015) (Fig. 6B). If MS inputs (and/or grid cells) convey self-motion cues or the output of path integration per se (as discussed above), these observations are consistent with a pivotal role of path integration in creating allocentric representations of novel environments based on perceptual inputs that are egocentrically experienced, as pointed out by Gallistel (1990) and studied in SLAM (Figs 5 and 6A).
By contrast, in a similar experiment in a novel but smaller environment, CA1 place fields developed normally (Brandon et al., 2014). A possible explanation for this difference is that in a small enclosure, boundary cells can look indistinguishable from grid cells (Savelli et al., 2008) (Fig. 3); thus, place field formation may be less vulnerable to the disruption of grid inputs if boundary cells are spared. Moreover, path integration is probably less critical for self-localization or map-building in small environments. In familiar environments both small (Koenig et al., 2011) and large (Wang et al., 2015), CA1 place cells also seem to function normally during MS inactivation [although the spatial firing of CA3 place cells can be disrupted compared with control animals (Mizumori et al., 1989)]. In a familiar environment, place cells may be able to keep their firing fields by relying on previously learned associations with landmarks (i.e. the known map), even if path integration is compromised by MS inactivation (similar to the Bayes filter with a given map described in Fig. 4, but in which the path integration term is suppressed). Thus, future work could experimentally investigate whether these place cells regress to plain sensory representations of allothetic cues/relationships while losing the very properties that have historically implicated place cells in a genuinely abstract representation of space and that may be obtained through path integration. As discussed at the beginning of this Review, the crucial distinction is between a spatial firing correlate owing to a sensory cue (the cell fires at a corner) versus a place representation that is not dependent on any particular sensory feature. For example, it is known that in normal animals, place cells remain intact in darkness and/or in the absence of salient landmarks (Markus et al., 1994; O'Keefe and Speakman, 1987; Quirk et al., 1990); they can decouple completely from the set of local and global landmarks yet maintain their own internal coherence (i.e. the map can rotate coherently relative to all allocentric cues) (Knierim et al., 1998, 1995); and the cells can maintain firing when any subset of landmarks are available, showing that they do not rely on the presence of a specific sensory cue (O'Keefe and Conway, 1978). It will be important to determine whether place fields unaffected by MS inactivation have similar properties. Earlier studies showed that CA3 and CA1 place fields were present after MS lesions (Miller and Best, 1980; Shapiro et al., 1989), but the cells were less stable in darkness than in control animals (Leutgeb and Mizumori, 1999), and were more controlled by local cues on an apparatus than by global landmarks on the wall (Miller and Best, 1980). Control by peripheral landmarks such as global cues or environmental boundaries is associated with directional orientation and the MEC system (Knierim and Hamilton, 2011; Neunuebel et al., 2013; Savelli et al., 2017; Zugaro et al., 2001), whereas control by local cues has been associated with the lateral entorhinal cortex system (Neunuebel et al., 2013). Lesions of the MEC that leave the lateral entorhinal cortex intact compromise CA1 place fields in relatively larger and novel environments (see fig. 2C of Hales et al., 2014), similar to the MS inactivation experiment discussed above (Wang et al., 2015). Thus, there is some evidence in the literature to suggest that the spatial firing that survives MS inactivation and grid cell disruption in smaller or familiar environments may be the result of spatial computations based on local landmarks (Deshmukh and Knierim, 2011; Wang et al., 2018) rather than path integration.
Are path-integration-like computations used in non-spatial domains?
The neural circuitry and dynamics that allow path integration computations in the hippocampal system may also explain a number of other phenomenological properties of neurons in this system. A growing body of evidence shows that hippocampal cells that represent locations when the animal is moving (i.e. place cells) can also represent time when the animal is running in place or is otherwise stationary but attentive (Gill et al., 2011; Kraus et al., 2015, 2013; MacDonald et al., 2011; Pastalkova et al., 2008; Salz et al., 2016). Under other conditions, the cells encode the distance traveled along a path (Gothard et al., 1996; Kraus et al., 2015, 2013; Ravassard et al., 2013). These three phenomenologically distinct representations (space, distance and time), encoded by the same population of cells, can in principle be explained by a single computational mechanism, as shown by Hasselmo (2007) using the oscillatory interference model of Burgess and colleagues (2007). Two fixed oscillators produce an interference pattern with a temporally constant beat frequency of its envelope (i.e. a time cell). However, if one of the oscillators was variable and its frequency was appropriately modulated by the running speed of the animal, the beat frequency of the interference pattern envelope would be a function of the distance traveled along a path (i.e. a distance cell). Further, if the variable oscillator was appropriately modulated by both speed and movement direction (a movement/velocity vector), the beat frequency of the envelope would be a function of location (i.e. a place cell or grid cell). Thus, three distinct phenomenological properties of the same set of neurons can be explained and unified based on computational principles, as the output of the network was determined only by changes to the inputs that adjust the frequency of the variable oscillator. Although Hasselmo (2007) used oscillatory interference models to demonstrate the principle, in theory the principle can be applied to attractor network models of place cells and grid cells as well. In support of this principle, the time cell properties of hippocampal neurons in a running wheel were disrupted when the MS inputs were silenced, in the same way as place cells were disrupted in large and novel environments (Wang et al., 2015).
The same arguments can unify the path integration models with the apparent representation by hippocampal cells of non-spatial variables. In one experiment, stationary rats were trained to listen to an auditory tone of monotonically increasing frequency and release a lever within a small frequency window (Aronov et al., 2017). The firing of different hippocampal cells was correlated with different auditory frequencies. Although one might interpret this result as a completely different computation than path integration, it is actually completely consistent with the ideas expressed above. When the rat is stationary, one can assume that the network is in the mode in which it acts like a clock (i.e. time cells). How does one explain the auditory frequency tuning? Just as when the system performs path integration during movement, with the external landmarks able to correct error in the path integration output and keep it calibrated with the external world, the only salient external cue in the auditory experiment is the varying frequency of the tone. The same neural plasticity that ties landmarks or scenes to spatial representations during path integration would allow the auditory cues to control the exact timing of the hippocampal cells after experience.
Does this reasoning imply that the auditory responses are somehow an artifact? Not at all. Our point here is that understanding path integration as a computational mechanism allows a unifying explanation of seemingly disparate neurophysiological phenomena. The hippocampal circuitry likely evolved to perform ethologically critical spatial mapping functions, and later became co-opted by the opportunistic process of natural selection to allow the animals to solve other tasks using the same neural hardware and computational principles. More precisely, a system that evolved to rapidly bind landmarks onto a path-integration-based mapping system could subsequently evolve to perform other rapid binding tasks of diverse external sensory input onto a sequential code. This way of thinking may be the link between disparate viewpoints of the roles of hippocampus in spatial mapping in rodents and declarative memory in humans (Burgess et al., 2002; Buzsáki, 2005; Buzsáki and Moser, 2013; Eichenbaum et al., 2016, 1999; Lisman, 1999; O'Keefe and Nadel, 1978).
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This study was funded by the National Institutes of Health [R01 NS039456; R01 NS102537; R21 NS095075; R01 MH079511]. Deposited in PMC for release after 12 months.
References
- Alexander A. S. and Nitz D. A. (2015). Retrosplenial cortex maps the conjunction of internal and external spaces. Nat. Neurosci. 18, 1143-1151. 10.1038/nn.4058 [DOI] [PubMed] [Google Scholar]
- Alexander A. S. and Nitz D. A. (2017). Spatially periodic activation patterns of retrosplenial cortex encode route sub-spaces and distance traveled. Curr. Biol. CB 27, 1551-1560.e4. 10.1016/j.cub.2017.04.036 [DOI] [PubMed] [Google Scholar]
- Arleo A., Déjean C., Allegraud P., Khamassi M., Zugaro M. B. and Wiener S. I. (2013). Optic flow stimuli update anterodorsal thalamus head direction neuronal activity in rats. J. Neurosci. 33, 16790-16795. 10.1523/JNEUROSCI.2698-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov D., Nevers R. and Tank D. W. (2017). Mapping of a non-spatial dimension by the hippocampal-entorhinal circuit. Nature 543, 719-722. 10.1038/nature21692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banino A., Barry C., Uria B., Blundell C., Lillicrap T., Mirowski P., Pritzel A., Chadwick M. J., Degris T., Modayil J. et al. (2018). Vector-based navigation using grid-like representations in artificial agents. Nature 557, 429-433. 10.1038/s41586-018-0102-6 [DOI] [PubMed] [Google Scholar]
- Barlow J. S. (1964). Inertial navigation as a basis for animal navigation. J. Theor. Biol. 6, 76-117. 10.1016/0022-5193(64)90067-0 [DOI] [PubMed] [Google Scholar]
- Barry C., Hayman R., Burgess N. and Jeffery K. J. (2007). Experience-dependent rescaling of entorhinal grids. Nat. Neurosci. 10, 682-684. 10.1038/nn1905 [DOI] [PubMed] [Google Scholar]
- Barry C., Lever C., Hayman R., Hartley T., Burton S., O'Keefe J., Jeffery K. and Burgess N. (2006). The boundary vector cell model of place cell firing and spatial memory. Rev. Neurosci. 17, 71-97. 10.1515/REVNEURO.2006.17.1-2.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Fonio E., Galili T., Havkin G. Z. and Golani I. (2011). Quantifying the buildup in extent and complexity of free exploration in mice. Proc. Natl. Acad. Sci. USA 108, 15580-15587. 10.1073/pnas.1014837108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner K. C., Milstein A. D., Grienberger C., Romani S. and Magee J. C. (2017). Behavioral time scale synaptic plasticity underlies CA1 place fields. Science 357, 1033-1036. 10.1126/science.aan3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes T. L., Dagslott N. C., Moser E. I. and Moser M.-B. (2018). Path integration in place cells of developing rats. Proc. Natl. Acad. Sci. USA 115, E1637-E1646. 10.1073/pnas.1719054115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H. T., Gupta K. and Zhang K. (2008). Conversion of a phase- to a rate-coded position signal by a three-stage model of theta cells, grid cells, and place cells. Hippocampus 18, 1239-1255. 10.1002/hipo.20509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H. T., Wu A. and Cong J. (2014). Oscillatory neurocomputing with ring attractors: a network architecture for mapping locations in space onto patterns of neural synchrony. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 369, 20120526 10.1098/rstb.2012.0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccara C. N., Sargolini F., Thoresen V. H., Solstad T., Witter M. P., Moser E. I. and Moser M.-B. (2010). Grid cells in pre- and parasubiculum. Nat. Neurosci. 13, 987-994. 10.1038/nn.2602 [DOI] [PubMed] [Google Scholar]
- Brandon M. P., Bogaard A. R., Libby C. P., Connerney M. A., Gupta K. and Hasselmo M. E. (2011). Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science 332, 595-599. 10.1126/science.1201652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon M. P., Koenig J., Leutgeb J. K. and Leutgeb S. (2014). New and distinct hippocampal place codes are generated in a new environment during septal inactivation. Neuron 82, 789-796. 10.1016/j.neuron.2014.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotons-Mas J. R., Schaffelhofer S., Guger C., O'Mara S. M. and Sanchez-Vives M. V. (2017). Heterogeneous spatial representation by different subpopulations of neurons in the subiculum. Neuroscience 343, 174-189. 10.1016/j.neuroscience.2016.11.042 [DOI] [PubMed] [Google Scholar]
- Burak Y. and Fiete I. R. (2009). Accurate path integration in continuous attractor network models of grid cells. PLoS Comput. Biol. 5, e1000291 10.1371/journal.pcbi.1000291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess C. P. and Burgess N. (2014). Controlling phase noise in oscillatory interference models of grid cell firing. J. Neurosci. 34, 6224-6232. 10.1523/JNEUROSCI.2540-12.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N., Maguire E. A. and O'Keefe J. (2002). The human hippocampus and spatial and episodic memory. Neuron 35, 625-641. 10.1016/S0896-6273(02)00830-9 [DOI] [PubMed] [Google Scholar]
- Burgess N., Barry C. and O'Keefe J. (2007). An oscillatory interference model of grid cell firing. Hippocampus 17, 801-812. 10.1002/hipo.20327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D., Barry C., Manson D. and Burgess N. (2015). Using grid cells for navigation. Neuron 87, 507-520. 10.1016/j.neuron.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. (2002). Theta oscillations in the hippocampus. Neuron 33, 325-340. 10.1016/S0896-6273(02)00586-X [DOI] [PubMed] [Google Scholar]
- Buzsáki G. (2005). Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus 15, 827-840. 10.1002/hipo.20113 [DOI] [PubMed] [Google Scholar]
- Buzsáki G. and Moser E. I. (2013). Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 16, 130-138. 10.1038/nn.3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne P., Becker S. and Burgess N. (2007). Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol. Rev. 114, 340-375. 10.1037/0033-295X.114.2.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacucci F., Lever C., Wills T. J., Burgess N. and O'Keefe J. (2004). Theta-modulated place-by-direction cells in the hippocampal formation in the rat. J. Neurosci. Off. J. Soc. Neurosci. 24, 8265-8277. 10.1523/JNEUROSCI.2635-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., King J. A., Burgess N. and O'Keefe J. (2013). How vision and movement combine in the hippocampal place code. Proc. Natl. Acad. Sci. USA 110, 378-383. 10.1073/pnas.1215834110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. (1986). A purely geometric module in the rat's spatial representation. Cognition 23, 149-178. 10.1016/0010-0277(86)90041-7 [DOI] [PubMed] [Google Scholar]
- Cheng K. (2008). Whither geometry? Troubles of the geometric module. Trends Cogn. Sci. 12, 355-361. 10.1016/j.tics.2008.06.004 [DOI] [PubMed] [Google Scholar]
- Cheng K. and Newcombe N. S. (2005). Is there a geometric module for spatial orientation? squaring theory and evidence. Psychon. Bull. Rev. 12, 1-23. 10.3758/BF03196346 [DOI] [PubMed] [Google Scholar]
- Cheung A. (2016). Probabilistic learning by rodent grid cells. PLoS Comput. Biol. 12, e1005165 10.1371/journal.pcbi.1005165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A., Ball D., Milford M., Wyeth G. and Wiles J. (2012). Maintaining a cognitive map in darkness: the need to fuse boundary knowledge with path integration. PLoS Comput. Biol. 8, e1002651 10.1371/journal.pcbi.1002651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couey J. J., Witoelar A., Zhang S.-J., Zheng K., Ye J., Dunn B., Czajkowski R., Moser M.-B., Moser E. I., Roudi Y et al. . et al. (2013). Recurrent inhibitory circuitry as a mechanism for grid formation. Nat. Neurosci. 16, 318-324. 10.1038/nn.3310 [DOI] [PubMed] [Google Scholar]
- Cressant A., Muller R. U. and Poucet B. (1997). Failure of centrally placed objects to control the firing fields of hippocampal place cells. J. Neurosci. Off. J. Soc. Neurosci. 17, 2531-2542. 10.1523/JNEUROSCI.17-07-02531.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva C. J. and Wei X.-X. (2018). Emergence of grid-like representations by training recurrent neural networks to perform spatial localization. ArXiv180307770 Cs Q-Bio Stat. [Google Scholar]
- Cullen K. E. and Taube J. S. (2017). Our sense of direction: progress, controversies and challenges. Nat. Neurosci. 20, 1465-1473. 10.1038/nn.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdikman D., Whitlock J. R., Tsao A., Fyhn M., Hafting T., Moser M.-B. and Moser E. I. (2009). Fragmentation of grid cell maps in a multicompartment environment. Nat. Neurosci. 12, 1325-1332. 10.1038/nn.2396 [DOI] [PubMed] [Google Scholar]
- Deshmukh S. S. and Knierim J. J. (2011). Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front. Behav. Neurosci. 5, 69 10.3389/fnbeh.2011.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh S. S. and Knierim J. J. (2013). Influence of local objects on hippocampal representations: landmark vectors and memory. Hippocampus 23, 253-267. 10.1002/hipo.22101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantaki M., Coletta S., Nasr K., Zeraati R., Laturnus S., Berens P., Preston-Ferrer P. and Burgalossi A. (2018). Manipulating hippocampal place cell activity by single-cell stimulation in freely moving mice. Cell Rep. 23, 32-38. 10.1016/j.celrep.2018.03.031 [DOI] [PubMed] [Google Scholar]
- Diehl G. W., Hon O. J., Leutgeb S. and Leutgeb J. K. (2017). Grid and nongrid cells in medial entorhinal cortex represent spatial location and environmental features with complementary coding schemes. Neuron 94, 83-92.e6. 10.1016/j.neuron.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G., Harris K. D. and Buzsáki G. (2003). Place representation within hippocampal networks is modified by long-term potentiation. Neuron 39, 843-853. 10.1016/S0896-6273(03)00465-3 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H., Dudchenko P., Wood E., Shapiro M. and Tanila H. (1999). The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron 23, 209-226. 10.1016/S0896-6273(00)80773-4 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H., Amaral D. G., Buffalo E. A., Buzsáki G., Cohen N., Davachi L., Frank L., Heckers S., Morris R. G. M., Moser E. I. et al. (2016). Hippocampus at 25. Hippocampus 26, 1238-1249. 10.1002/hipo.22616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam D. and Golani I. (1989). Home base behavior of rats (Rattus norvegicus) exploring a novel environment. Behav. Brain Res. 34, 199-211. 10.1016/S0166-4328(89)80102-0 [DOI] [PubMed] [Google Scholar]
- Erdem U. M. and Hasselmo M. (2012). A goal-directed spatial navigation model using forward trajectory planning based on grid cells. Eur. J. Neurosci. 35, 916-931. 10.1111/j.1460-9568.2012.08015.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem U. M. and Hasselmo M. E. (2014). A biologically inspired hierarchical goal directed navigation model. J. Physiol. Paris 108, 28-37. 10.1016/j.jphysparis.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdem U. M., Milford M. J. and Hasselmo M. E. (2015). A hierarchical model of goal directed navigation selects trajectories in a visual environment. Neurobiol. Learn. Mem. 117, 109-121. 10.1016/j.nlm.2014.07.003 [DOI] [PubMed] [Google Scholar]
- Etienne A. S. and Jeffery K. J. (2004). Path integration in mammals. Hippocampus 14, 180-192. 10.1002/hipo.10173 [DOI] [PubMed] [Google Scholar]
- Etienne A. S., Maurer R. and Séguinot V. (1996). Path integration in mammals and its interaction with visual landmarks. J. Exp. Biol. 199, 201-209. [DOI] [PubMed] [Google Scholar]
- Evans T., Bicanski A., Bush D. and Burgess N. (2016). How environment and self-motion combine in neural representations of space. J. Physiol. 594, 6535-6546. 10.1113/JP270666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattahi M., Sharif F., Geiller T. and Royer S. (2018). Differential representation of landmark and self-motion information along the CA1 radial axis: self-motion generated place fields shift toward landmarks during septal inactivation. J. Neurosci. 38, 6766-6788 10.1523/JNEUROSCI.3211-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent K. and Wehner R. (1985). Oceili: a celestial compass in the desert ant Cataglyphis. Science 228, 192-194. 10.1126/science.228.4696.192 [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Las L. and Ulanovsky N. (2016). 3-D maps and compasses in the brain. Annu. Rev. Neurosci. 39, 171-196. 10.1146/annurev-neuro-070815-013831 [DOI] [PubMed] [Google Scholar]
- Frank L. M., Stanley G. B. and Brown E. N. (2004). Hippocampal plasticity across multiple days of exposure to novel environments. J. Neurosci. Off. J. Soc. Neurosci. 24, 7681-7689. 10.1523/JNEUROSCI.1958-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzius M., Sprekeler H. and Wiskott L. (2007). Slowness and sparseness lead to place, head-direction, and spatial-view cells. PLoS Comput. Biol. 3, e166 10.1371/journal.pcbi.0030166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann F., Justus D., Sosulina L., Kaneko H., Beutel T., Friedrichs D., Schoch S., Schwarz M. K., Fuhrmann M. and Remy S. (2015). Locomotion, theta oscillations, and the speed-correlated firing of hippocampal neurons are controlled by a medial septal glutamatergic circuit. Neuron 86, 1253-1264. 10.1016/j.neuron.2015.05.001 [DOI] [PubMed] [Google Scholar]
- Fuhs M. C. and Touretzky D. S. (2006). A spin glass model of path integration in rat medial entorhinal cortex. J. Neurosci. Off. J. Soc. Neurosci. 26, 4266-4276. 10.1523/JNEUROSCI.4353-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhn M., Hafting T., Treves A., Moser M.-B. and Moser E. I. (2007). Hippocampal remapping and grid realignment in entorhinal cortex. Nature 446, 190-194. 10.1038/nature05601 [DOI] [PubMed] [Google Scholar]
- Gallistel C. R. (1990). The Organization of Learning. Cambridge, MA: MIT Press. [Google Scholar]
- Gil M., Ancau M., Schlesiger M. I., Neitz A., Allen K., De Marco R. J. and Monyer H. (2018). Impaired path integration in mice with disrupted grid cell firing. Nat. Neurosci. 21, 81-91. 10.1038/s41593-017-0039-3 [DOI] [PubMed] [Google Scholar]
- Gill P. R., Mizumori S. J. Y. and Smith D. M. (2011). Hippocampal episode fields develop with learning. Hippocampus 21, 1240-1249. 10.1002/hipo.20832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golani I., Benjamini Y. and Eilam D. (1993). Stopping behavior: constraints on exploration in rats (Rattus norvegicus). Behav. Brain Res. 53, 21-33. 10.1016/S0166-4328(05)80263-3 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Sulser A., Parthier D., Candela A., McClure C., Pastoll H., Garden D., Sürmeli G. and Nolan M. F. (2014). GABAergic projections from the medial septum selectively inhibit interneurons in the medial entorhinal cortex. J. Neurosci. Off. J. Soc. Neurosci. 34, 16739-16743. 10.1523/JNEUROSCI.1612-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge J. P. and Taube J. S. (1997). Interaction between the postsubiculum and anterior thalamus in the generation of head direction cell activity. J. Neurosci. Off. J. Soc. Neurosci. 17, 9315-9330. 10.1523/JNEUROSCI.17-23-09315.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard K. M., Skaggs W. E. and McNaughton B. L. (1996). Dynamics of mismatch correction in the hippocampal ensemble code for space: interaction between path integration and environmental cues. J. Neurosci. 16, 8027-8040. 10.1523/JNEUROSCI.16-24-08027.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard K. M., Hoffman K. L., Battaglia F. P. and McNaughton B. L. (2001). Dentate gyrus and ca1 ensemble activity during spatial reference frame shifts in the presence and absence of visual input. J. Neurosci. Off. J. Soc. Neurosci. 21, 7284-7292. 10.1523/JNEUROSCI.21-18-07284.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., Adachi A., Shah K. K., Hirokawa J. D., Magani P. S. and Maimon G. (2017). A neural circuit architecture for angular integration in Drosophila. Nature 546, 101-106. 10.1038/nature22343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guanella A., Kiper D. and Verschure P. (2007). A model of grid cells based on a twisted torus topology. Int. J. Neural Syst. 17, 231-240. 10.1142/S0129065707001093 [DOI] [PubMed] [Google Scholar]
- Hafting T., Fyhn M., Molden S., Moser M.-B. and Moser E. I. (2005). Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801-806. 10.1038/nature03721 [DOI] [PubMed] [Google Scholar]
- Hales J. B., Schlesiger M. I., Leutgeb J. K., Squire L. R., Leutgeb S. and Clark R. E. (2014). Medial entorhinal cortex lesions only partially disrupt hippocampal place cells and hippocampus-dependent place memory. Cell Rep. 9, 893-901. 10.1016/j.celrep.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle K., Ganguli S. and Giocomo L. M. (2015). Environmental boundaries as an error correction mechanism for grid cells. Neuron 86, 827-839. 10.1016/j.neuron.2015.03.039 [DOI] [PubMed] [Google Scholar]
- Hardcastle K., Maheswaranathan N., Ganguli S. and Giocomo L. M. (2017). A multiplexed, heterogeneous, and adaptive code for navigation in medial entorhinal cortex. Neuron 94, 375-387.e7. 10.1016/j.neuron.2017.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T., Burgess N., Lever C., Cacucci F. and O'Keefe J. (2000). Modeling place fields in terms of the cortical inputs to the hippocampus. Hippocampus 10, 369-379. <369::AID-HIPO3>3.0.CO;2-0 [DOI] [PubMed] [Google Scholar]
- Harvey C. D., Collman F., Dombeck D. A. and Tank D. W. (2009). Intracellular dynamics of hippocampal place cells during virtual navigation. Nature 461, 941-946. 10.1038/nature08499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo M. E. (2007). Arc length coding by interference of theta frequency oscillations may underlie context-dependent hippocampal unit data and episodic memory function. Learn. Mem. 14, 782-794. 10.1101/lm.686607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo M. E. (2008). Grid cell mechanisms and function: contributions of entorhinal persistent spiking and phase resetting. Hippocampus 18, 1213-1229. 10.1002/hipo.20512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo M. E. and Brandon M. P. (2012). A model combining oscillations and attractor dynamics for generation of grid cell firing. Front. Neural Circuits 6 10.3389/fncir.2012.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo M. E., Giocomo L. M. and Zilli E. A. (2007). Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons. Hippocampus 17, 1252-1271. 10.1002/hipo.20374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S., Narendra A. and Cheung A. (2018). Principles of insect path integration. Curr. Biol. CB 28, R1043-R1058. 10.1016/j.cub.2018.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines D. J. and Whishaw I. Q. (2005). Home bases formed to visual cues but not to self-movement (dead reckoning) cues in exploring hippocampectomized rats. Eur. J. Neurosci. 22, 2363-2375. 10.1111/j.1460-9568.2005.04412.x [DOI] [PubMed] [Google Scholar]
- Hinman J. R., Brandon M. P., Climer J. R., Chapman G. W. and Hasselmo M. E. (2016). Multiple running speed signals in medial entorhinal cortex. Neuron 91, 666-679. 10.1016/j.neuron.2016.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C. A., Newcombe N. S. and Shipley T. F. (2018). Move to learn: Integrating spatial information from multiple viewpoints. Cognition 178, 7-25. 10.1016/j.cognition.2018.05.003 [DOI] [PubMed] [Google Scholar]
- Hoydal O. A., Skytoen E. R., Moser M.-B., Moser E. I. (2018). Object-vector coding in the medial entorhinal cortex. bioRxiv 286286. [DOI] [PubMed] [Google Scholar]
- Jacob P.-Y., Gordillo-Salas M., Facchini J., Poucet B., Save E. and Sargolini F. (2017). Medial entorhinal cortex and medial septum contribute to self-motion-based linear distance estimation. Brain Struct. Funct. 222, 2727-2742. 10.1007/s00429-017-1368-4 [DOI] [PubMed] [Google Scholar]
- Jankowski M. M. and O'Mara S. M. (2015). Dynamics of place, boundary and object encoding in rat anterior claustrum. Front. Behav. Neurosci. 9, 250 10.3389/fnbeh.2015.00250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski M. M., Passecker J., Islam M. N., Vann S., Erichsen J. T., Aggleton J. P. and O'Mara S. M. (2015). Evidence for spatially-responsive neurons in the rostral thalamus. Front. Behav. Neurosci. 9, 256 10.3389/fnbeh.2015.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar R. P., Madhav M. S., Savelli F., Blair H. T., Cowan N. J. and Knierim J. J. (2019). Recalibration of path integration in hippocampal place cells. Nature (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeewajee A., Barry C., O'Keefe J. and Burgess N. (2008). Grid cells and theta as oscillatory interference: Electrophysiological data from freely moving rats. Hippocampus 18, 1175-1185. 10.1002/hipo.20510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus D., Dalügge D., Bothe S., Fuhrmann F., Hannes C., Kaneko H., Friedrichs D., Sosulina L., Schwarz I., Elliott D. A. et al. (2017). Glutamatergic synaptic integration of locomotion speed via septoentorhinal projections. Nat. Neurosci. 20, 16-19. 10.1038/nn.4447 [DOI] [PubMed] [Google Scholar]
- Kanitscheider I. and Fiete I. (2017). Making our way through the world: Towards a functional understanding of the brain's spatial circuits. Curr. Opin. Syst. Biol. 3, 186-194. 10.1016/j.coisb.2017.04.008 [DOI] [Google Scholar]
- Kanter B. R., Lykken C. M., Avesar D., Weible A., Dickinson J., Dunn B., Borgesius N. Z., Roudi Y. and Kentros C. G. (2017). A novel mechanism for the grid-to-place cell transformation revealed by transgenic depolarization of medial entorhinal cortex layer II. Neuron 93, 1480-1492.e6. 10.1016/j.neuron.2017.03.001 [DOI] [PubMed] [Google Scholar]
- Keene C. S., Bladon J., McKenzie S., Liu C. D., O'Keefe J. and Eichenbaum H. (2016). Complementary functional organization of neuronal activity patterns in the perirhinal, lateral entorhinal, and medial entorhinal cortices. J. Neurosci. Off. J. Soc. Neurosci. 36, 3660-3675. 10.1523/JNEUROSCI.4368-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinath A. T., Epstein R. A. and Balasubramanian V. (2018). Environmental deformations dynamically shift the grid cell spatial metric. eLife7. 10.7554/eLife.38169 [DOI]
- Kim S. S., Rouault H., Druckmann S. and Jayaraman V. (2017). Ring attractor dynamics in the Drosophila central brain. Science 356, 849-853. 10.1126/science.aal4835 [DOI] [PubMed] [Google Scholar]
- King C., Recce M. and O'Keefe J. (1998). The rhythmicity of cells of the medial septum/diagonal band of Broca in the awake freely moving rat: relationships with behaviour and hippocampal theta. Eur. J. Neurosci. 10, 464-477. 10.1046/j.1460-9568.1998.00026.x [DOI] [PubMed] [Google Scholar]
- Knierim J. J. (2002). The Path-Integration Properties of Hippocampal Place Cells, in: The Neural Basis of Navigation, pp. 41-58. Boston, MA: Springer. [Google Scholar]
- Knierim J. J. and Hamilton D. A. (2011). Framing spatial cognition: neural representations of proximal and distal frames of reference and their roles in navigation. Physiol. Rev. 91, 1245-1279. 10.1152/physrev.00021.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim J. J. and Zhang K. (2012). Attractor dynamics of spatially correlated neural activity in the limbic system. Annu. Rev. Neurosci. 35, 267-285. 10.1146/annurev-neuro-062111-150351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim J. J., Kudrimoti H. S. and McNaughton B. L. (1995). Place cells, head direction cells, and the learning of landmark stability. J. Neurosci. Off. J. Soc. Neurosci. 15, 1648-1659. 10.1523/JNEUROSCI.15-03-01648.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim J. J., Kudrimoti H. S. and McNaughton B. L. (1998). Interactions between idiothetic cues and external landmarks in the control of place cells and head direction cells. J. Neurophysiol. 80, 425-446. 10.1152/jn.1998.80.1.425 [DOI] [PubMed] [Google Scholar]
- Koenig J., Linder A. N., Leutgeb J. K. and Leutgeb S. (2011). The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science 332, 592-595. 10.1126/science.1201685 [DOI] [PubMed] [Google Scholar]
- Kramer D. L. and McLaughlin R. L. (2001). The behavioral ecology of intermittent locomotion. Am. Zool. 41, 137-153. 10.1093/icb/41.2.137 [DOI] [Google Scholar]
- Kraus B. J., Robinson R. J., White J. A., Eichenbaum H. and Hasselmo M. E. (2013). Hippocampal “time cells”: time versus path integration. Neuron 78, 1090-1101. 10.1016/j.neuron.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus B. J., Brandon M. P., Robinson R. J., Connerney M. A., Hasselmo M. E. and Eichenbaum H. (2015). During running in place, grid cells integrate elapsed time and distance run. Neuron 88, 578-589. 10.1016/j.neuron.2015.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropff E. and Treves A. (2008). The emergence of grid cells: intelligent design or just adaptation? Hippocampus 18, 1256-1269. 10.1002/hipo.20520 [DOI] [PubMed] [Google Scholar]
- Kropff E., Carmichael J. E., Moser M.-B. and Moser E. I. (2015). Speed cells in the medial entorhinal cortex. Nature 523, 419-424. 10.1038/nature14622 [DOI] [PubMed] [Google Scholar]
- Krupic J., Bauza M., Burton S., Barry C. and O'Keefe J. (2015). Grid cell symmetry is shaped by environmental geometry. Nature 518, 232-235. 10.1038/nature14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupic J., Bauza M., Burton S. and O'Keefe J. (2016). Framing the grid: effect of boundaries on grid cells and navigation. J. Physiol. 594, 6489-6499. 10.1113/JP270607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubie J. L. and Fenton A. A. (2012). Linear look-ahead in conjunctive cells: an entorhinal mechanism for vector-based navigation. Front. Neural Circuits 6, 20 10.3389/fncir.2012.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers B. (2000). The spatial semantic hierarchy. Artif. Intell. 119, 191-233. 10.1016/S0004-3702(00)00017-5 [DOI] [Google Scholar]
- Kuipers B., Modayil J., Beeson P., MacMahon M. and Savelli F. (2004). Local metrical and global topological maps in the hybrid spatial semantic hierarchy. In 2004 IEEE International Conference on Robotics and Automation, 2004, pp. 4845-4851. IEEE [Google Scholar]
- Leutgeb S. and Mizumori S. J. Y. (1999). Excitotoxic septal lesions result in spatial memory deficits and altered flexibility of hippocampal single-unit representations. J. Neurosci. 19, 6661-6672. 10.1523/JNEUROSCI.19-15-06661.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb S. and Mizumori S. J. Y. (2002). Context-specific spatial representations by lateral septal cells. Neuroscience 112, 655-663. 10.1016/S0306-4522(02)00101-X [DOI] [PubMed] [Google Scholar]
- Lever C., Burton S., Jeewajee A., O'Keefe J. and Burgess N. (2009). Boundary vector cells in the subiculum of the hippocampal formation. J. Neurosci. Off. J. Soc. Neurosci. 29, 9771-9777. 10.1523/JNEUROSCI.1319-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E. (1999). Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate–CA3 interactions. Neuron 22, 233-242. 10.1016/S0896-6273(00)81085-5 [DOI] [PubMed] [Google Scholar]
- MacDonald C. J., Lepage K. Q., Eden U. T. and Eichenbaum H. (2011). Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron 71, 737-749. 10.1016/j.neuron.2011.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory C. S., Hardcastle K., Bant J. S. and Giocomo L. M. (2018). Grid scale drives the scale and long-term stability of place maps. Nat. Neurosci. 21, 270-282. 10.1038/s41593-017-0055-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus E. J., Barnes C. A., McNaughton B. L., Gladden V. L. and Skaggs W. E. (1994). Spatial information content and reliability of hippocampal CA1 neurons: effects of visual input. Hippocampus 4, 410-421. 10.1002/hipo.450040404 [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Knierim J. J., Wilson M. A. (1995). Vector encoding and the vestibular foundations of spatial cognition: neurophysiological and computational mechanisms In The Cognitive Neurosciences (ed. Gazzaniga M. S.), pp. 585-595. Cambridge, MA: The MIT Press. [Google Scholar]
- McNaughton B. L., Barnes C. A., Gerrard J. L., Gothard K., Jung M. W., Knierim J. J., Kudrimoti H., Qin Y., Skaggs W. E., Suster M. et al. (1996). Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J. Exp. Biol. 199, 173-185. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Battaglia F. P., Jensen O., Moser E. I. and Moser M.-B. (2006). Path integration and the neural basis of the “cognitive map”. Nat. Rev. Neurosci. 7, 663-678. 10.1038/nrn1932 [DOI] [PubMed] [Google Scholar]
- Miao C., Cao Q., Ito H. T., Yamahachi H., Witter M. P., Moser M.-B. and Moser E. I. (2015). Hippocampal remapping after partial inactivation of the medial entorhinal cortex. Neuron 88, 590-603. 10.1016/j.neuron.2015.09.051 [DOI] [PubMed] [Google Scholar]
- Miller V. M. and Best P. J. (1980). Spatial correlates of hippocampal unit activity are altered by lesions of the fornix and endorhinal cortex. Brain Res. 194, 311-323. 10.1016/0006-8993(80)91214-7 [DOI] [PubMed] [Google Scholar]
- Mimica B., Dunn B. A., Tombaz T., Bojja V. P. T. N. C. S. and Whitlock J. R. (2018). Efficient cortical coding of 3D posture in freely behaving rats. Science 362, 584-589. 10.1126/science.aau2013 [DOI] [PubMed] [Google Scholar]
- Mizumori S. J., McNaughton B. L., Barnes C. A. and Fox K. B. (1989). Preserved spatial coding in hippocampal CA1 pyramidal cells during reversible suppression of CA3c output: evidence for pattern completion in hippocampus. J. Neurosci. 9, 3915-3928. 10.1523/JNEUROSCI.09-11-03915.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco J. D., Knierim J. J. and Zhang K. (2011). Sensory feedback, error correction, and remapping in a multiple oscillator model of place-cell activity. Front. Comput. Neurosci. 5, 39 10.3389/fncom.2011.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco J. D., Rao G., Roth E. D. and Knierim J. J. (2014). Attentive scanning behavior drives one-trial potentiation of hippocampal place fields. Nat. Neurosci. 17, 725-731. 10.1038/nn.3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco J. D., De Guzman R. M., Blair H. T. and Zhang K. (2019). Spatial synchronization codes from coupled rate-phase neurons. PLoS Comput. Biol. 15, e1006741 10.1371/journal.pcbi.1006741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E. I. and Moser M.-B. (2008). A metric for space. Hippocampus 18, 1142-1156. 10.1002/hipo.20483 [DOI] [PubMed] [Google Scholar]
- Moser E. I., Moser M.-B. and McNaughton B. L. (2017). Spatial representation in the hippocampal formation: a history. Nat. Neurosci. 20, 1448-1464. 10.1038/nn.4653 [DOI] [PubMed] [Google Scholar]
- Neunuebel J. P., Yoganarasimha D., Rao G. and Knierim J. J. (2013). Conflicts between local and global spatial frameworks dissociate neural representations of the lateral and medial entorhinal cortex. J. Neurosci. 33, 9246-9258. 10.1523/JNEUROSCI.0946-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D. A. (2006). Tracking route progression in the posterior parietal cortex. Neuron 49, 747-756. 10.1016/j.neuron.2006.01.037 [DOI] [PubMed] [Google Scholar]
- Nitz D. A. (2009). Parietal cortex, navigation, and the construction of arbitrary reference frames for spatial information. Neurobiol. Learn. Mem. 91, 179-185. 10.1016/j.nlm.2008.08.007 [DOI] [PubMed] [Google Scholar]
- O'Keefe J. (1976). Place units in the hippocampus of the freely moving rat. Exp. Neurol. 51, 78-109. 10.1016/0014-4886(76)90055-8 [DOI] [PubMed] [Google Scholar]
- O'Keefe J. and Burgess N. (1996). Geometric determinants of the place fields of hippocampal neurons. Nature 381, 425-428. 10.1038/381425a0 [DOI] [PubMed] [Google Scholar]
- O'Keefe J. and Conway D. H. (1978). Hippocampal place units in the freely moving rat: why they fire where they fire. Exp. Brain Res. 31, 573-590. 10.1007/BF00239813 [DOI] [PubMed] [Google Scholar]
- O'Keefe J. and Dostrovsky J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171-175. 10.1016/0006-8993(71)90358-1 [DOI] [PubMed] [Google Scholar]
- O'Keefe J. and Nadel L. (1978). The Hippocampus as a Cognitive Map. Oxford: Clarendon Press. [Google Scholar]
- O'Keefe J. and Speakman A. (1987). Single unit activity in the rat hippocampus during a spatial memory task. Exp. Brain Res. 68, 1-27. 10.1007/BF00255230 [DOI] [PubMed] [Google Scholar]
- Pastalkova E., Itskov V., Amarasingham A. and Buzsáki G. (2008). Internally generated cell assembly sequences in the rat hippocampus. Science 321, 1322-1327. 10.1126/science.1159775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoll H., Solanka L., van Rossum M. C. W. and Nolan M. F. (2013). Feedback inhibition enables θ-nested γ oscillations and grid firing fields. Neuron 77, 141-154. 10.1016/j.neuron.2012.11.032 [DOI] [PubMed] [Google Scholar]
- Peyrache A., Schieferstein N. and Buzsáki G. (2017). Transformation of the head-direction signal into a spatial code. Nat. Commun. 8, 1752 10.1038/s41467-017-01908-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G. J., Muller R. U. and Kubie J. L. (1990). The firing of hippocampal place cells in the dark depends on the rat's recent experience. J. Neurosci. Off. J. Soc. Neurosci. 10, 2008-2017. 10.1523/JNEUROSCI.10-06-02008.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudies F., Brandon M. P., Chapman G. W. and Hasselmo M. E. (2015). Head direction is coded more strongly than movement direction in a population of entorhinal neurons. Brain Res. 1621, 355-367. 10.1016/j.brainres.2014.10.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravassard P., Kees A., Willers B., Ho D., Aharoni D. A., Cushman J., Aghajan Z. M. and Mehta M. R. (2013). Multisensory control of hippocampal spatiotemporal selectivity. Science 340, 1342-1346. 10.1126/science.1232655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish A. D. (1999). Beyond the Cognitive Map: from Place Cells to Episodic Memory. MIT Press. [Google Scholar]
- Redish A. D. and Touretzky D. S. (1997). Cognitive maps beyond the hippocampus. Hippocampus 7, 15-35. [DOI] [PubMed] [Google Scholar]
- Rueckemann J. W., DiMauro A. J., Rangel L. M., Han X., Boyden E. S. and Eichenbaum H. (2016). Transient optogenetic inactivation of the medial entorhinal cortex biases the active population of hippocampal neurons. Hippocampus 26, 246-260. 10.1002/hipo.22519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salz D. M., Tiganj Z., Khasnabish S., Kohley A., Sheehan D., Howard M. W. and Eichenbaum H. (2016). Time cells in hippocampal area CA3. J. Neurosci. 36, 7476-7484. 10.1523/JNEUROSCI.0087-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargolini F., Fyhn M., Hafting T., McNaughton B. L., Witter M. P., Moser M.-B. and Moser E. I. (2006). Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312, 758-762. 10.1126/science.1125572 [DOI] [PubMed] [Google Scholar]
- Save E., Cressant A., Thinus-Blanc C. and Poucet B. (1998). Spatial firing of hippocampal place cells in blind rats. J. Neurosci. Off. J. Soc. Neurosci. 18, 1818-1826. 10.1523/JNEUROSCI.18-05-01818.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelli F., Yoganarasimha D. and Knierim J. J. (2008). Influence of boundary removal on the spatial representations of the medial entorhinal cortex. Hippocampus 18, 1270-1282. 10.1002/hipo.20511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelli F., Luck J. D. and Knierim J. J. (2017). Framing of grid cells within and beyond navigation boundaries. eLife 6, 493 10.7554/eLife.21354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. L., Simon D. K., Olton D. S., Gage F. H., Nilsson O. and Björklund A. (1989). Intrahippocampal grafts of fetal basal forebrain tissue alter place fields in the hippocampus of rats with fimbria-fornix lesions. Neuroscience 32, 1-18. 10.1016/0306-4522(89)90103-6 [DOI] [PubMed] [Google Scholar]
- Sharp P. E. (1999). Subicular place cells expand or contract their spatial firing pattern to fit the size of the environment in an open field but not in the presence of barriers: comparison with hippocampal place cells. Behav. Neurosci. 113, 643-662. 10.1037/0735-7044.113.4.643 [DOI] [PubMed] [Google Scholar]
- Sharp P. E., Blair H. T., Etkin D. and Tzanetos D. B. (1995). Influences of vestibular and visual motion information on the spatial firing patterns of hippocampal place cells. J. Neurosci. Off. J. Soc. Neurosci. 15, 173-189. 10.1523/JNEUROSCI.15-01-00173.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipston-Sharman O., Solanka L. and Nolan M. F. (2016). Continuous attractor network models of grid cell firing based on excitatory–inhibitory interactions. J. Physiol. 594, 6547-6557. 10.1113/JP270630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si B. and Treves A. (2013). A model for the differentiation between grid and conjunctive units in medial entorhinal cortex. Hippocampus 23, 1410-1424. 10.1002/hipo.22194 [DOI] [PubMed] [Google Scholar]
- Skaggs W. E., Knierim J. J., Kudrimoti H. S. and McNaughton B. L. (1995). A model of the neural basis of the rat's sense of direction. Adv. Neural Inf. Process. Syst. 7, 173-180. [PubMed] [Google Scholar]
- Solstad T., Boccara C. N., Kropff E., Moser M.-B. and Moser E. I. (2008). Representation of geometric borders in the entorhinal cortex. Science 322, 1865-1868. 10.1126/science.1166466 [DOI] [PubMed] [Google Scholar]
- Stemmler M., Mathis A. and Herz A. V. M. (2015). Connecting multiple spatial scales to decode the population activity of grid cells. Sci. Adv. 1, e1500816 10.1126/science.1500816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensola H., Stensola T., Solstad T., Frøland K., Moser M.-B. and Moser E. I. (2012). The entorhinal grid map is discretized. Nature 492, 72-78. 10.1038/nature11649 [DOI] [PubMed] [Google Scholar]
- Stensola T., Stensola H., Moser M.-B. and Moser E. I. (2015). Shearing-induced asymmetry in entorhinal grid cells. Nature 518, 207-212. 10.1038/nature14151 [DOI] [PubMed] [Google Scholar]
- Takamura Y., Tamura R., Zhou T. L., Kobayashi T., Tran A. H., Eifuku S. and Ono T. (2006). Spatial firing properties of lateral septal neurons. Hippocampus 16, 635-644. 10.1002/hipo.20196 [DOI] [PubMed] [Google Scholar]
- Taube J. S. (2007). The head direction signal: origins and sensory-motor integration. Annu. Rev. Neurosci. 30, 181-207. 10.1146/annurev.neuro.29.051605.112854 [DOI] [PubMed] [Google Scholar]
- Tennant S. A., Fischer L., Garden D. L. F., Gerlei K. Z., Martinez-Gonzalez C., McClure C., Wood E. R. and Nolan M. F. (2018). Stellate cells in the medial entorhinal cortex are required for spatial learning. Cell Rep. 22, 1313-1324. 10.1016/j.celrep.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrazas A., Krause M., Lipa P., Gothard K. M., Barnes C. A. and McNaughton B. L. (2005). Self-motion and the hippocampal spatial metric. J. Neurosci. Off. J. Soc. Neurosci. 25, 8085-8096. 10.1523/JNEUROSCI.0693-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. M., Berkowitz L. E. and Clark B. J. (2018). Behavioral and neural subsystems of rodent exploration. Learn. Motiv. 61, 3-15. 10.1016/j.lmot.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrun S., Burgard W. and Fox D. (2005). Probabilistic Robotics. MIT Press. [Google Scholar]
- Tingley D. and Buzsáki G. (2018). Transformation of a spatial map across the hippocampal-lateral septal circuit. Neuron 98, 1229-1242.e5. 10.1016/j.neuron.2018.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi L., Chiandetti C., Pecchia T., Sovrano V. A. and Vallortigara G. (2012). From natural geometry to spatial cognition. Neurosci. Biobehav. Rev. 36, 799-824. 10.1016/j.neubiorev.2011.12.007 [DOI] [PubMed] [Google Scholar]
- Touretzky D. S., Weisman W. E., Fuhs M. C., Skaggs W. E., Fenton A. A. and Muller R. U. (2005). Deforming the hippocampal map. Hippocampus 15, 41-55. 10.1002/hipo.20029 [DOI] [PubMed] [Google Scholar]
- Turner-Evans D., Wegener S., Rouault H., Franconville R., Wolff T., Seelig J. D., Druckmann S. and Jayaraman V. (2017). Angular velocity integration in a fly heading circuit. eLife 6, e04577 10.7554/eLife.23496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdapilleta E., Si B. and Treves A. (2017). Selforganization of modular activity of grid cells. Hippocampus 27, 1204-1213. 10.1002/hipo.22765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. G., Hines D. J. and Whishaw I. Q. (2002). Quantification of a single exploratory trip reveals hippocampal formation mediated dead reckoning. J. Neurosci. Methods 113, 131-145. 10.1016/S0165-0270(01)00489-7 [DOI] [PubMed] [Google Scholar]
- Wang Y., Romani S., Lustig B., Leonardo A. and Pastalkova E. (2015). Theta sequences are essential for internally generated hippocampal firing fields. Nat. Neurosci. 18, 282-288. 10.1038/nn.3904 [DOI] [PubMed] [Google Scholar]
- Wang C., Chen X., Lee H., Deshmukh S. S., Yoganarasimha D., Savelli F. and Knierim J. J. (2018). Egocentric coding of external items in the lateral entorhinal cortex. Science 362, 945-949. 10.1126/science.aau4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S. N. and Sprekeler H. (2018). Learning place cells, grid cells and invariances with excitatory and inhibitory plasticity. eLife 7, 287 10.7554/eLife.34560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner R. and Srinivasan M. V. (1981). Searching behaviour of desert ants, genus Cataglyphis (Formicidae, Hymenoptera). J. Comp. Physiol. 142, 315-338. 10.1007/BF00605445 [DOI] [Google Scholar]
- Welday A. C., Shlifer I. G., Bloom M. L., Zhang K. and Blair H. T. (2011). Cosine directional tuning of theta cell burst frequencies: evidence for spatial coding by oscillatory interference. J. Neurosci. 31, 16157-16176. 10.1523/JNEUROSCI.0712-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. R., Pfuhl G., Dagslott N., Moser M.-B. and Moser E. I. (2012). Functional split between parietal and entorhinal cortices in the rat. Neuron 73, 789-802. 10.1016/j.neuron.2011.12.028 [DOI] [PubMed] [Google Scholar]
- Wilber A. A., Clark B. J., Forster T. C., Tatsuno M. and McNaughton B. L. (2014). Interaction of egocentric and world-centered reference frames in the rat posterior parietal cortex. J. Neurosci. 34, 5431-5446. 10.1523/JNEUROSCI.0511-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder R. M., Peck J. R. and Taube J. S. (2015). Visual landmark information gains control of the head direction signal at the lateral mammillary nuclei. J. Neurosci. 35, 1354-1367. 10.1523/JNEUROSCI.1418-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K., Buice M. A., Barry C., Hayman R., Burgess N. and Fiete I. R. (2013). Specific evidence of low-dimensional continuous attractor dynamics in grid cells. Nat. Neurosci. 16, 1077-1084. 10.1038/nn.3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K. (1996). Representation of spatial orientation by the intrinsic dynamics of the head-direction cell ensemble: a theory. J. Neurosci. 16, 2112-2126. 10.1523/JNEUROSCI.16-06-02112.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Schönfeld F., Wiskott L. and Manahan-Vaughan D. (2014). Spatial representations of place cells in darkness are supported by path integration and border information. Front. Behav. Neurosci. 8, 222 10.3389/fnbeh.2014.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugaro M. B., Berthoz A. and Wiener S. I. (2001). Background, but not foreground, spatial cues are taken as references for head direction responses by rat anterodorsal thalamus neurons. J. Neurosci. 21, RC154 10.1523/JNEUROSCI.21-14-j0001.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]