Fig. 6.
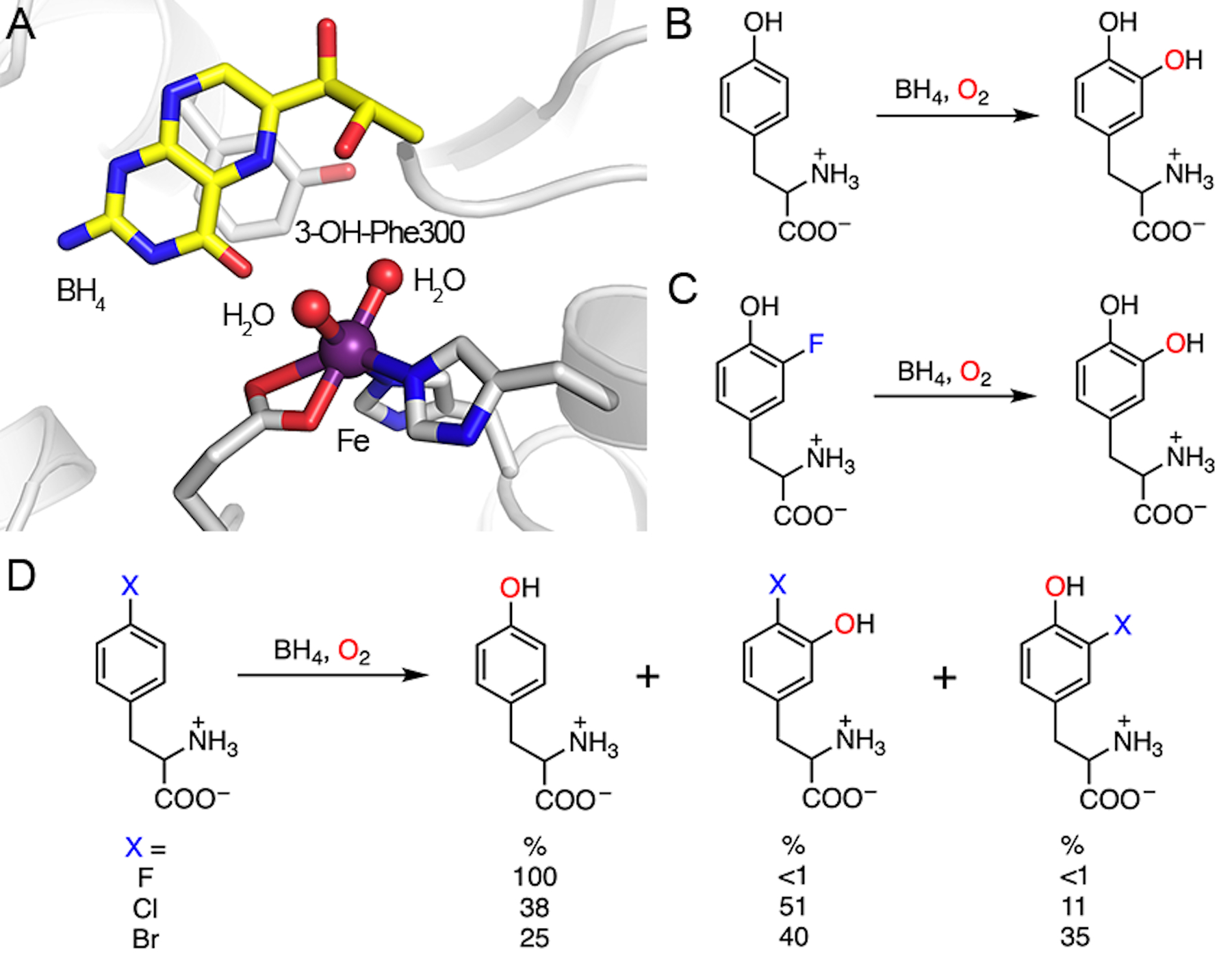
Active site of nonheme TyrH and the demonstrated reactions. (A) Crystal structure of nonheme TyrH in complex with BH4. Phe300 is self-hydroxylated to 3-OH-Phe300. PDB entry: 2TOH. (B) Natural hydroxylation of tyrosine. (C) Defluorination of 3-fluorotyrosine. (D) Dehalogenation of 4-halo-phenylalanine. 4-Fluorophenylalanine (X = F) only yields one product, namely, tyrosine. Other halogen substitutions (X = Cl, Br) afford multiple products.
