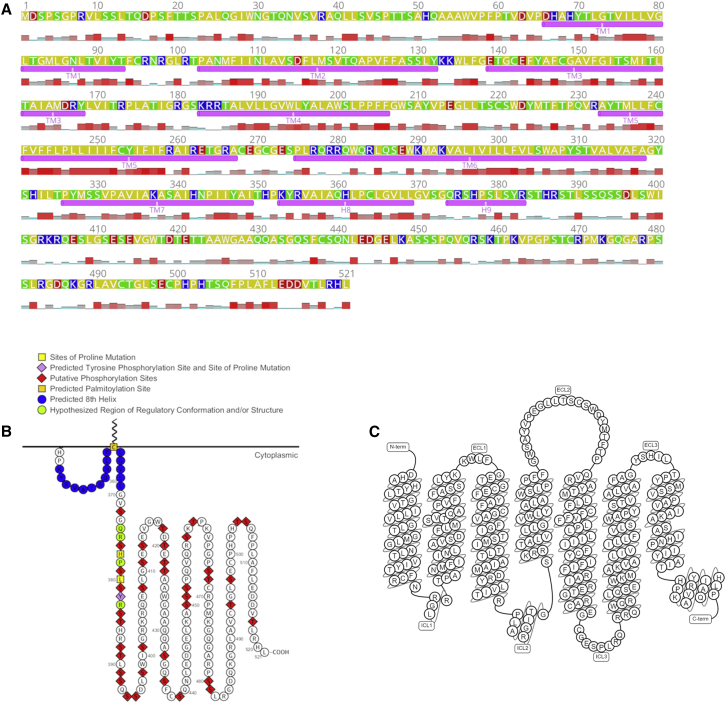
Figure 1.
Mouse melanopsin’s amino acid sequence. Mouse melanopsin is 521 amino acids long, with a long C-terminus domain that is 171 amino acids long. (A) Transmembrane and cytoplasmic helices are annotated below the amino acid sequence. Amino acid property is denoted by the color of the residue: yellow, nonpolar; green, polar and noncharged; blue, positively charged; and red, negatively charged. Hydrophobicity at each residue is plotted below the amino acid sequence; taller and red bars indicate a high level of hydrophobicity at that position. Identities of residues possessing secondary structures are based on prediction from the homology mode generated (see Fig. 2). Figure was generated using Geneious software (63). (B) 2D schematic of mouse melanopsin C terminus depicting functionally significant residues. Figure was made using Protter software (64). (C) 2D snakeplot of mouse melanopsin’s secondary structure. Figure is from GPCRdb, using Swiss-Prot for secondary structure prediction (65). Note that the amino acids labeled as transmembrane helices in (A), not (C), correspond to identities of the amino acids in the homology model in Fig. 2.