FIG 7.
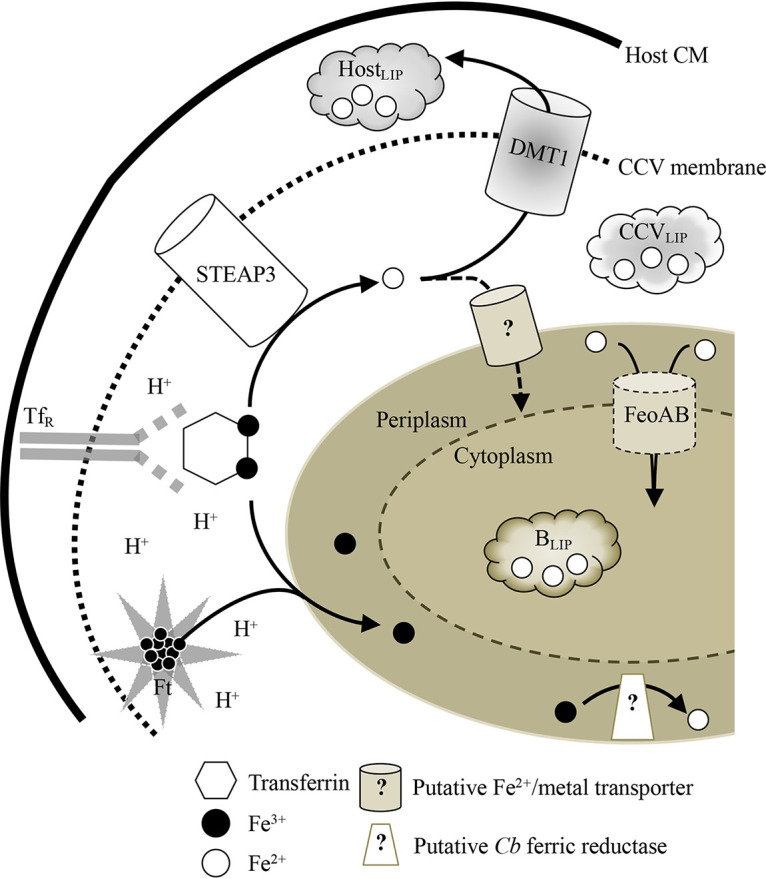
Working model for the role of iron in C. burnetii replication and viability. C. burnetii acquires molecular iron through acid degradation of iron-binding proteins (e.g., transferrin [Tf] and ferritin [Ft]) after uptake into the acidic CCV. Ferric iron released from iron-containing proteins is likely reduced within the CCV via the host enzyme STEAP3, delivered to the CCV upon fusion with endosomes. Transportation of Fe3+ into the periplasm of Coxiella would require conversion of Fe3+ to Fe2+ via a Coxiella-specific ferric reductase in order for C. burnetii to transport Fe2+ via FeoAB to the cytosol for replication and viability. Alternatively, Fe3+ reduced via STEAP3 to Fe2+ can be (i) shuttled outside the CCV via the DMT1 transporter and maintained in the host labile iron pool (HostLIP), (ii) remain within the CCV in a similar labile iron pool (CCVLIP), or (iii) be actively transported into the Coxiella periplasm by a putative Fe2+/metal transporter. Once in the periplasmic space, Fe2+ can be directly acquired via FeoAB for use in bacterial replication and viability or reside within a putative bacterial labile iron pool (BLIP).
