Abstract
A valued marine oil rich in omega-3 lipids and natural astaxanthin is obtained with remarkably high yield (up to 5 wt %) extending to pink shrimp waste (head and carapace) using the approach to extract fish oil from fish processing byproducts using d-limonene. Biobased limonene is an excellent solvent for both unsaturated lipids and astaxanthin-based carotenoids preventing oxidative degradation during the extraction cycle including solvent separation at 85 °C. Explaining the deep red color of the shrimp oil obtained, computational simulation suggests that d-limonene is also a good solvent for natural astaxanthin abundant in shrimp.
Introduction
Driven by high and increasing demand, the global catch of shrimp currently exceeds 4.5 million tonnes per year.1 In 2018, the global farmed shrimp production increased by 6% reaching almost 3.5 million tonnes, when wild shrimp production amounted to about 1 million tonnes.1
Depending on the processing conditions and on the species, the shell (body carapace) and the head of shrimp after the meat removal amount to around 50% of the overall shrimp weight.2
Since the early 1990s, plentiful research has been devoted to develop chemical2 and biotechnological3 (biotransformation with enzymes and microorganisms) to recover the valued chitin (coated with calcium carbonate) and carotenoids (mainly astaxanthin) comprising the latter shrimp waste.
Chitin-based commercial products are mostly used for wound dressing and as nutraceuticals, with industrial applications “still limited because of issues regarding optimization of mechanical and biological properties according to intended application”.4
Shrimp waste also contains lipids originating both from the cephalothorax and eyestalk and from the meat residues in the shell. Since 2015, one company in the port city of Cuxhaven, Germany, successfully uses shrimp waste to obtain valued fish oil and fish protein concentrates for human consumption.5
In general, the current lack of effective green chemistry methods to extract the valued natural products present in shrimp waste creates the conditions for environmental hazard and increases production costs. If thrown into the sea, the shrimp waste with its high protein content threatens populations of endangered species and impacts the product quality of coastal aquaculture.6
Current proper disposal adds to production costs because shrimp processing companies need to pay for disposal via incineration or sanitary landfilling after stabilization. Finally, after shrimp processing for meat removal, highly perishable shrimp waste rapidly decays (within an hour in tropical climates)6 leading to formation of toxic and bad-smelling biogenic amines.
In brief, there is a need to develop a low cost and easily scalable method to extract valued natural products from the shrimp waste. We now report the discovery of such new method in high and global demand by extending to shrimp waste the recently discovered method to extract fish oil from anchovy filleting waste using d-limonene as the green biosolvent.7
A marine oil rich in omega-3 lipids and natural astaxanthin is easily obtained with high yields (up to 5 wt %). Explaining the deep red color of the oil thereby obtained by advanced computational simulation suggests that biobased, citrus-derived limonene is also a good solvent for natural astaxanthin, a well known neuroprotective agent, abundant in shrimp.
Results and Discussion
A sample of deep-water pink shrimp waste (Parapenaeus longirostris) obtained from a fishery in Palermo, Sicily, was separated in three parts: carapace (33 g), heads (33 g), and head with carapace (33 g).
Each aliquot was added with 66 g of d-limonene (Acros Organics, 96% purity), and the resulting mixture was ground using an electric blender to obtain a pink puree (Figure 1). After grinding, the mixture was transferred to a beaker. The beaker was covered with an aluminum foil, and the mixture left under magnetic stirring for 24 h at room temperature.
Figure 1.

Sample of P. longirostris carapace waste prior (left) and after homogenization with an electric blender (right). Photograph courtesy of A. Scurria. Copyright 2020.
The mixture was thus centrifuged at 10,000 rpm (at 4 °C) for 15 min, after which the supernatant was transferred to the evaporation flask of a rotary evaporator to remove and fully recover the biosolvent under reduced pressure (26 mbar) at 85 °C (Figure 2).
Figure 2.
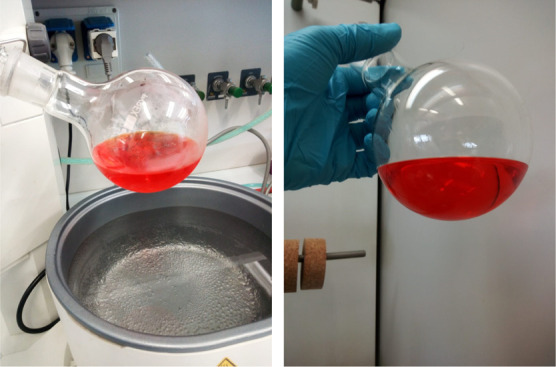
Extract from P. longirostris carapace in limonene prior to evaporation of the biosolvent. Photograph courtesy of C. Lino. Copyright 2020.
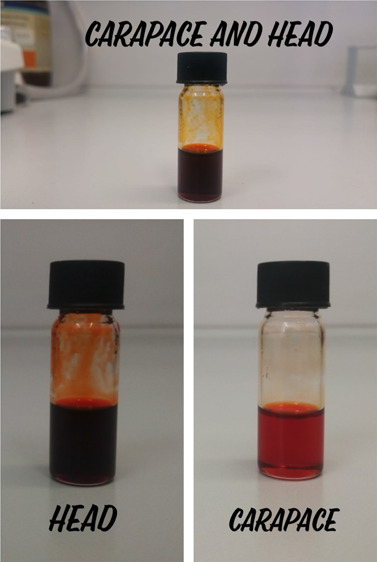
Rapid evaporation of limonene under reduced pressure leaves a significant amount of a deep red or red oil containing plentiful carotenoids depending on the biological sample extracted: carapace, 370 mg (1.12% yield); head, 1.65 g (5% yield); head with carapace, 1.38 g (4.18% yield).
The red color is because of astaxanthin and its astaxanthin monoester and astaxanthin diester, well known to impart the pink and red color to shrimp.8 In agreement with the fact that the highest levels of carotenoids are found in the cephalothorax,8 the oil from the head and carapace has a much more intense color (Figure 3).
Figure 3.

Different oils obtained via solid–liquid extraction with limonene from different parts of P. longirostris waste. Photograph courtesy of C. Lino. Copyright 2020.
The thin-layer chromatographic separation of the carotenoids in all the oils yields several bands, including the three bands corresponding to astaxanthin, astaxanthin monoester, and astaxanthin diester. The quantitative analysis of carotenoids will be reported shortly.
The fatty acid composition of the oil obtained from the heads is displayed in Table 1.
Table 1. Fatty Acids Identified in the Shrimp Oil Obtained from P. longirostris Heads.
| acid (in lipid numbers) | retention time (min) | abundance (%) |
|---|---|---|
| myristic (14:0) | 15.29 | 2.58 |
| 13-methylmyristic | 16.24 | 0.35 |
| pentadecanoic (15:0) | 17.19 | 1.22 |
| palmitic (16:0) | 19.39 | 18.92 |
| palmitoleic (16:1) | 19.78 | 0.79 |
| 9-hexadecenoic | 20.40 | 5.29 |
| 14-methylhexadecanoic | 20.87 | 0.48 |
| 7-methyl-6-hexadecenoic | 21.33 | 0.38 |
| margaric (17:0) | 21.52 | 1.25 |
| eptadecenoic (17:1) | 22.59 | 0.75 |
| isostearic (18:0) | 22.73 | 0.53 |
| stearic (18:0) | 24.00 | 5.95 |
| oleic (18:1, n-9) | 25.08 | 15.6 |
| trans-13-octadecenoic (18:1) | 25.23 | 3.74 |
| linoleic (18:2, n-6) | 26.74 | 1.38 |
| cis-10-nonadecenoic (19:1, n-9) | 27.55 | 0.19 |
| linolenic (18:3, n-3) | 28.93 | 1.14 |
| cis-11-eicosenoic (20:1, n-9) | 30.03 | 1.72 |
| γ-linolenic (18:3, n-6) | 30.24 | 1.40 |
| cis-11-14-eicosadienoic (20:2, n-6) | 31.91 | 1.56 |
| arachidonic (20:4, n-6) | 34.1 | 4.57 |
| eicosapentenoic (20:5, n-3) | 36.48 | 10.28 |
| 8-11-14-docosatrienoic (22:3, n-8) | 40.25 | 2.18 |
| nervonic (24:1, n-9) | 40.50 | 1.04 |
| docosapentaenoic (22:5, n-3) | 41.77 | 1.28 |
| docosahexaenoic (22:6, n-3) | 42.67 | 15.41 |
| saturated fatty acids | 30.8% | |
| unsaturated fatty acids | 69.2% | |
| monounsaturated fatty acids | 30% | |
| polyunsaturated fatty acids | 39.2% | |
Table 2 shows the lipid profile of the oil obtained from the head and carapace waste mixture.
Table 2. Fatty Acids Identified in the Shrimp Oil Obtained from P. longirostris Head and Carapace Waste.
| acid (in lipid numbers) | retention time (min) | abundance (%) |
|---|---|---|
| myristic (14:0) | 15.29 | 2.70 |
| 13-methylmyristic | 16.24 | 0.43 |
| pentadecanoic (15:0) | 17.19 | 1.16 |
| palmitic (16:0) | 19.35 | 19.44 |
| palmitoleic (16:1) | 19.77 | 0.76 |
| 9-hexadecenoic | 20.38 | 5.30 |
| 14-methylhexadecanoic | 20.87 | 0.40 |
| 7-methyl-6-hexadecenoic | 21.33 | 0.54 |
| margaric (17:0) | 21.52 | 1.26 |
| eptadecenoic (17:1) | 22.58 | 0.64 |
| isostearic (18:0) | 22.70 | 0.54 |
| stearic (18:0) | 23.95 | 5.73 |
| oleic (18:1, n-9) | 25.04 | 15.35 |
| trans-13-octadecenoic (18:1) | 25.20 | 3.78 |
| linoleic (18:2, n-6) | 26.72 | 1.47 |
| cis-10-nonadecenoic (19:1, n-9) | 27.55 | 0.33 |
| linolenic (18:3, n-3) | 28.91 | 1.35 |
| cis-11-eicosenoic (20:1, n-9) | 30.01 | 1.90 |
| γ-linolenic (18:3, n-6) | 30.24 | 1.53 |
| cis-11-14-eicosadienoic (20:2, n-6) | 31.89 | 1.52 |
| arachidonic (20:4, n-6) | 34.07 | 4.35 |
| eicosapentenoic (20:5, n-3) | 36.46 | 10.23 |
| 8-11-14-docosatrienoic (22:3, n-8) | 40.22 | 1.82 |
| nervonic (24:1, n-9) | 40.48 | 0.57 |
| docosapentaenoic (22:5, n-3) | 41.76 | 1.09 |
| docosahexaenoic (22:6, n-3) | 42.63 | 15.80 |
| saturated fatty acids | 31.3% | |
| unsaturated fatty acids | 67.3% | |
| monounsaturated fatty acids | 29.6% | |
| polyunsaturated fatty acids | 37.7% | |
Table 3 shows the lipid profile of the oil obtained from carapace only.
Table 3. Fatty Acids Identified in the Shrimp Oil Obtained from the P. longirostris Carapace Waste.
| acid (in lipid numbers) | retention time (min) | abundance (%) |
|---|---|---|
| myristic (14:0) | 15.36 | 2.44 |
| palmitic (16:0) | 19.26 | 21.56 |
| margaric (17:0) | 21.54 | 2.52 |
| stearic (18:0) | 23.88 | 7.99 |
| oleic (18:1, n-9) | 24.91 | 16.37 |
| trans-13-octadecenoic (18:1) | 25.14 | 4.91 |
| linoleic (18:2, n-6) | 26.75 | 2.46 |
| linolenic (18:3; n-3) | 28.96 | 2.21 |
| cis-11-eicosenoic (20:1, n-9) | 30.01 | 2.16 |
| γ-linolenic (18:3, n-6) | 30.29 | 1.91 |
| cis-11-14-eicosadienoic (20:2, n-6) | 31.91 | 3.71 |
| arachidonic (20:4, n-6) | 34.06 | 7.44 |
| eicosapentenoic (20:5, n-3) | 36.38 | 9.84 |
| docosahexaenoic (22:6, n-3) | 42.50 | 14.47 |
| saturated fatty acids | 34.51% | |
| unsaturated fatty acids | 65.48% | |
| monounsaturated fatty acids | 23.44% | |
| polyunsaturated fatty acids | 42.04% | |
The first remarkable finding is that waste shrimp oil has a high content of unsaturated acids exceeding 65% levels independent of the body part of the shrimp processing waste. Amid unsaturated acids, polyunsaturated fatty acids (PUFA) are predominant, varying between 37.7% of all fatty acids in the oil obtained from the head and carapace waste, and a maximum of 42.04% in the oil obtained from the shrimp shell waste only.
However, the major (and diriment) difference amid the oils is that whereas 14 fatty acids only are present in the carapace oil, almost twice as much (26) fatty acid moieties are present in the oils from the head alone (Table 1) and from head and thorax together (Table 2).
In each case, the levels of health-beneficial docosahexaenoic acid (DHA) and eicosapentenoic acid (EPA) omega-3 lipids are high, varying between a minimum of 14.47% in the thorax oil through a maximum of 15.80% in the combined shrimp waste oil and between 9.84% in the thorax oil and a maximum of 10.28% in the head oil for EPA.
Pointing to its presence in the head of the pink shrimp, one important omega-3 lipid is present in the head (1.28%) and in the head + thorax oil (1.09%), namely docosapentaenoic acid, which is the most abundant omega-3 long-chain PUFA present in the brain and more abundant than EPA in human milk, that could be particularly beneficial for early-life development and neuroprotection in the elderly.9
The trans isomer of C18 acid 13-octadecanoic acid, found in 0.22% amount in the seed oil of Phaleria macrocarpa medicinal plant,10 and rapidly adsorbed by human plasma, is particularly abundant in pink shrimp’s thorax oil (4.91%) and also in the head (3.74%) and head + thorax (3.78) oils.
Rare MUFA 11-eicosenoic acid (gondoic acid, 1.72 and 1.90%) and nervonic acid (1.04 and 0.47%) lately associated with mortality11 are present in oils extracted from shrimp waste containing the animal heads.
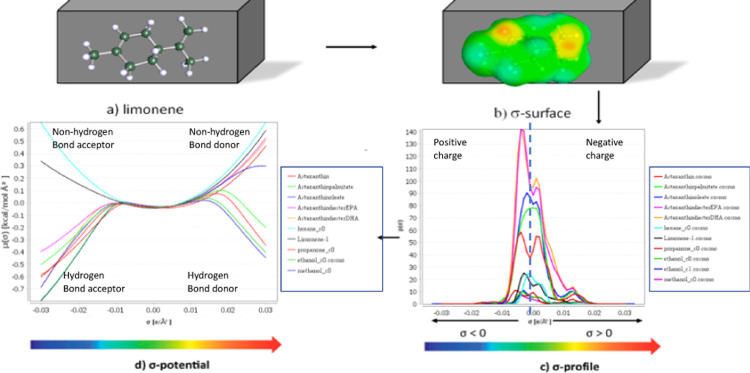
The color of the present oils is chiefly because of natural astaxanthin, a carotenoid with numerous health-beneficial properties today widely used as nutraceutical and food ingredients.12 We decided therefore to perform a theoretical study via a computational method (COSMO-RS, software package from COSMOlogic, Germany) in order to predict and rationalize solubility of different astaxanthin derivatives present in the shrimp in different solvents, including limonene.
In the simulation (Figure 4), all selected molecules are embedded into virtual conductors simulated in the first step by the COSMO model, where the molecule induces a polarization charge density (σ) on its surface (a good local descriptor of the molecular surface polarity).
Figure 4.
σ-potential of limonene and of all astaxanthin-based solutes studied (left) and the three-dimensional σ-profile surface modeling (right).
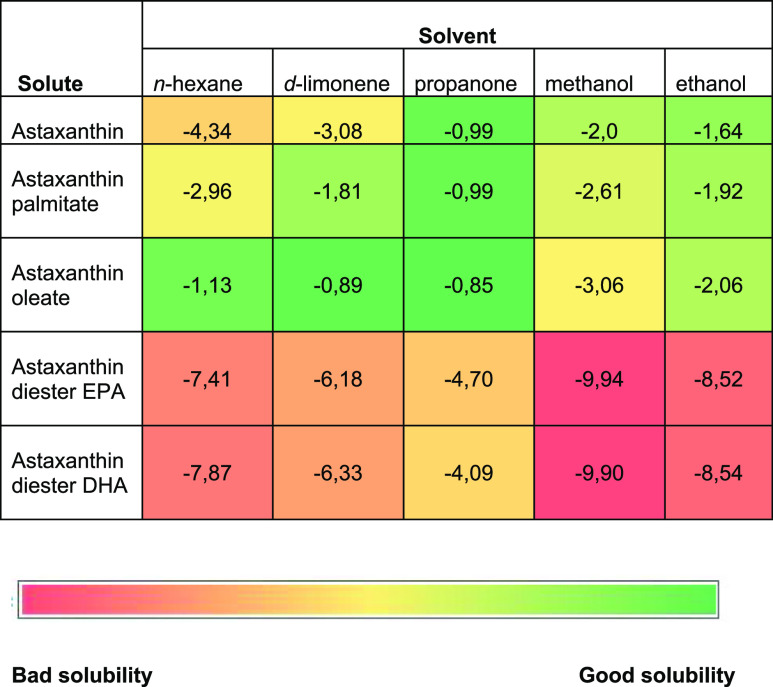
Table 4 shows the resulting predictions of different astaxanthin-based compounds, such as astaxanthin, astaxanthin palmitate, astaxanthin oleate, astaxanthin diester of EPA, and astaxanthin diester of DHA, in n-hexane, limonene, propanone, methanol, and ethanol, respectively.
Table 4. Predicted Solubility of Astaxanthin and Selected Mono and Diesters in d-Limonene at 25 °Ca.
Values are given as log10(xsolub).
Results of the simulations expressed in log10(xsolub) show a much higher theoretical solubility at 25 °C of the compounds, whose color in the color-based solubility bar, as shown in Table 4, approaches green. The simulation shows that d-limonene is a better solvent than n-hexane for astaxanthin derivatives, with log10(x solubility) values each time lower.
Limonene is also a better solvent than ethanol and methanol, except for free astaxanthin. The best solvent for the selected astaxanthin-based compounds is acetone (propanone), but biobased limonene is a good alternative in terms of solubilization power.
Bad Solubility Good Solubility
The fact that ethanol is an excellent extraction solvent for astaxanthin (but not so for the omega-3 diesters) was recently experimentally confirmed by scholars in China who, under the optimal conditions of the solid–liquid ratio 1:7, T = 50 °C, and 20 min extraction time, obtained a 50.32 μg/g astaxanthin yield from fresh Pandalus borealis shells, eventually obtaining an astaxanthin extract with 0.34% titre in astaxanthin.13
Our computational results are also in agreement with the fact that a considerably higher yield of 72.42 μg/g was obtained by extracting the carotenoid from the shells of deep-water pink shrimp (P. longirostris) with acetone in the dark at 4 °C.14 Besides excluding light, the latter process required the use of 20 mg of synthetic antioxidant butylated hydroxytoluene (BHT) to prevent astaxanthin oxidative degradation. Even though approved since more than 70 years in small amounts as an antioxidant food additive, BHT is toxic and has a moderate to high bioaccumulation potential.15
The new extraction process reported in the present study does not require to exclude light neither commands the use of synthetic antioxidants. Limonene, indeed, is an antioxidant terpene,16 whose use in the extraction of shrimp oil from shrimp waste intrinsically prevents the oxidative degradation of both natural astaxanthin and polyunsaturated omega-3 fatty acids abundant in marine lipids. Limonene, furthermore, is a powerful antibacterial agent16 inhibiting the growth of most pathogenic bacteria causing fish food poisoning.17
Conclusions
The findings reported in this study establish a new, low cost, and simple route to a marine oil rich in omega-3 and in natural astaxanthin directly made available from biowaste globally available in over 2.2 million tonnes per year.1
Natural astaxanthin is a powerful antioxidant and a neuroprotective agent.18 Accordingly, several nutraceutical products based on naturally stable astaxanthin for healthy aging have been commercialized.19
It is also likely that new synergistic effects between omega-3 lipids and astaxanthin, already observed for the antioxidant power when low concentrations of astaxanthin are combined with DHA or EPA,20 might be identified in the newly available shrimp oil extracted with this novel method. We will report soon the outcomes of the first biological experiments.
Analyzing the technical and economic feasibility of fish oil production from anchovy leftovers using d-limonene as the only solvent at room temperature, we have lately shown that the process provides clear economic and environmental benefits in face of relatively small capital and operational expenses.21
Isolated as the main component of orange oil obtained at orange juice factories prior to the fruit squeezing for juice production,22 biobased limonene used as the key enabler of the extraction process is easily and virtually entirely recovered via evaporation under reduced pressure. This affords a circular economy process with significant potential for practical application because the cost of the initial purchase of expensive citrus limonene16 is readily recovered via recycle of the biosolvent in several consecutive extraction cycles.21
Finally, the lipid- and carotenoid-free solid residue after extraction contains plentiful chitin and proteins whose decomposition has been inhibited, thanks to prolonged contact with antimicrobial limonene.17 In a subsequent study, we will show how to effectively process this solid (95% in weight of the original shrimp waste) separating the proteic fraction from chitin, to eventually make both of them available for further value-added uses.
Experimental Section
The fatty acid analysis was carried out via gas chromatography–mass spectrometry (GC–MS) on the fatty acid methyl esters (FAMEs) of the oils. A 100 mg of sample of each shrimp oil was evaporated with a flux of nitrogen gas to remove residual traces of d-limonene. The oil sample was trans-esterified by adding a 50 μL of aliquot of a MeOK solution (2 M) previously prepared by dissolving KOH (extrapure pellets, Merck) in methanol (≥99,8%, Sigma-Aldrich).
The resulting FAME was mixed with 500 μL of n-hexane, prior to injection for the GC–MS analysis employing the technique in scan mode. In detail, the analysis was carried out using Trace1310 coupled with ISQ and TriPlus autosampler (all by Thermo Fisher). The instrument was equipped with a TR FAME capillary column (100 m × 0.25 mm, 0.25 μm) using 5.5 ultrapure helium (99.9995% purity) as the gas carrier.
The injection was in split mode (1:10), and the temperature was set at 250 °C. The oven temperature started at 100 °C (held for 0.2 min), increased first until 150 °C (with a ramp temperature of 6 °C/min), and then until 240 °C (ramp temperature 2 °C/min) held for 5 min. A certified reference material (Supelco 37 Component FAME Mix) was used for both the qualitative and quantitative analyses.
The retention times and molecular fragment mass data obtained were processed using the instrument software. Each measurement was repeated three times. The fatty acids were identified from the corresponding FAMEs by critical comparison with mass spectral data from NIST/EPA/NIH Mass Spectral Library 2005.
Acknowledgments
This study is dedicated to Fabio Granata, former vice President of Sicily, for all he has done to establish Sicily’s Superintendency of the Sea in collaboration with the late Professor Sebastiano Tusa.
The authors declare no competing financial interest.
References
- Sackton J.Global Shrimp Production. 2018 International Congress on World Fisheries Production, 6th Conxemar-Fao Congress, Vigo, Spain, October 1, 2018.
- Kandra P.; Challa M. M.; Jyothi H. K. P. Efficient use of shrimp waste: present and future trends. Appl. Microbiol. Biotechnol. 2012, 93, 17–29. 10.1007/s00253-011-3651-2. [DOI] [PubMed] [Google Scholar]
- Mao X.; Guo N.; Sun J.; Xue C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Cleaner Prod. 2017, 143, 814–823. 10.1016/j.jclepro.2016.12.042. [DOI] [Google Scholar]
- Yadav M.; Goswami P.; Paritosh K.; Kumar M.; Pareek N.; Vivekanand V. Seafood waste: a source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8. 10.1186/s40643-019-0243-y. [DOI] [Google Scholar]
- www.lipromar.de/en/lip/food/ (last accessed June 9, 2020).
- Kandra P.; Challa M. M.; Jyothi H. K. P. Efficient use of shrimp waste: present and future trends. Appl. Microbiol. Biotechnol. 2012, 93, 17–29. 10.1007/s00253-011-3651-2. [DOI] [PubMed] [Google Scholar]
- Ciriminna R.; Scurria A.; Avellone G.; Pagliaro M. A Circular Economy Approach to Fish Oil Extraction. ChemistrySelect 2019, 4, 5106–5109. 10.1002/slct.201900851. [DOI] [Google Scholar]
- The astaxanthin composition in shrimp includes three astaxanthin stereoisomers in free and esterified form, with significant variations depending on the species, and significantly different for same body component of different species. See:Su F.; Huang B.; Liu J. The carotenoids of shrimps (Decapoda: Caridea and Dendrobranchiata) cultured in China. J. Crustacean Biol. 2018, 38, 523–530. 10.1093/jcbiol/ruy049. [DOI] [Google Scholar]
- Drouin G.; Rioux V.; Legrand P. The n-3 docosapentaenoic acid (DPA): A new player in the n-3 long chain polyunsaturated fatty acid family. Biochimie 2019, 159, 36–48. 10.1016/j.biochi.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Azmir J.; Zaidul I. S. M.; Sharif K. M.; Uddin M. S.; Jahurul M. H. A.; Jinap S.; Hajeb P.; Mohamed A. Supercritical carbon dioxide extraction of highly unsaturated oil from Phaleria macrocarpa seed. Food Res. Int. 2014, 65, 394–400. 10.1016/j.foodres.2014.06.049. [DOI] [Google Scholar]
- Delgado G. E.; Krämer B. K.; Lorkowski S.; März W.; von Schacky C.; Kleber M. E. Individual omega-9 monounsaturated fatty acids and mortality-The Ludwigshafen Risk and Cardiovascular Health Study. J. Clin. Lipidol. 2017, 11, 126–135. 10.1016/j.jacl.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Brendler T.; Williamson E. M. Astaxanthin: How much is too much? A safety review. Phytother. Res. 2019, 33, 3090–3111. 10.1002/ptr.6514. [DOI] [PubMed] [Google Scholar]
- Hu J.; Lu W.; Lv M.; Wang Y.; Ding R.; Wang L. Extraction and purification of astaxanthin from shrimp shells and the effects of different treatments on its content. Rev. Bras. Farmacogn. 2019, 29, 24–29. 10.1016/j.bjp.2018.11.004. [DOI] [Google Scholar]
- Sila A.; Ayed-Ajmi Y.; Sayari N.; Nasri M.; Martinez-Alvarez O.; Bougatef A. Antioxidant and Anti-proliferative Activities of Astaxanthin Extracted from the Shell Waste of Deep-water Pink Shrimp (Parapenaeus longirostris). Nat. Prod. J. 2013, 3, 82–89. 10.2174/2210315511303020002. [DOI] [Google Scholar]
- Nieva-Echevarría B.; Manzanos M. J.; Goicoechea E.; Guillén M. D. 2,6-Di-Tert-Butyl-Hydroxytoluene and Its Metabolites in Foods. Compr. Rev. Food Sci. Food Saf. 2015, 14, 67–80. 10.1111/1541-4337.12121. [DOI] [PubMed] [Google Scholar]
- Ciriminna R.; Lomeli-Rodriguez M.; Demma Carà P.; Lopez-Sanchez J. A.; Pagliaro M. Limonene: A Versatile Chemical of the Bioeconomy. Chem. Commun. 2014, 50, 15288–15296. 10.1039/c4cc06147k. [DOI] [PubMed] [Google Scholar]
- Pathirana H. N. K. S.; Wimalasena S. H. M. P.; De Silva B. C. J.; Hossain S.; Heo G.-J. Antibacterial activity of lime (Citrus aurantifolia) essential oil and limonene against fish pathogenic bacteria isolated from cultured olive flounder (Paralichthys olivaceus). Fish. Aquat. Life 2018, 26, 131–139. 10.2478/aopf-2018-0014. [DOI] [Google Scholar]
- Fakhri S.; Aneva I. Y.; Farzaei M. H.; Sobarzo-Sánchez E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 2019, 24, 2640. 10.3390/molecules24142640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivo L.Astaxanthin: An Antioxidant Powerhouse, Nutraceuticals World, April 25, 2018. www.nutraceuticalsworld.com/contents/view_online-exclusives/2018-04-25/astaxanthin-an-antioxidant-powerhouse/ (last accessed June 9, 2020).
- Saw C. L. L.; Yang A. Y.; Guo Y.; Kong A.-N. T. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the NRF2-ARE pathway. Food Chem. Toxicol. 2013, 62, 869–875. 10.1016/j.fct.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Ciriminna R.; Scurria A.; Fabiano-Tixier A.-S.; Lino C.; Avellone G.; Chemat F.; Pagliaro M. Omega-3 Extraction from Anchovy Fillet Leftovers with Limonene: Chemical, Economic, and Technical Aspects. ACS Omega 2019, 4, 15359–15363. 10.1021/acsomega.9b01168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriminna R.; Meneguzzo F.; Pagliaro M. In Orange Oil In Green Pesticides Handbook: Essential Oils for Pest Control; Nollet L. M. L., Rathore H. S., Eds.; Routledge: London, 2017; Chapter 15, pp 291–302. [Google Scholar]