Fig. 5.
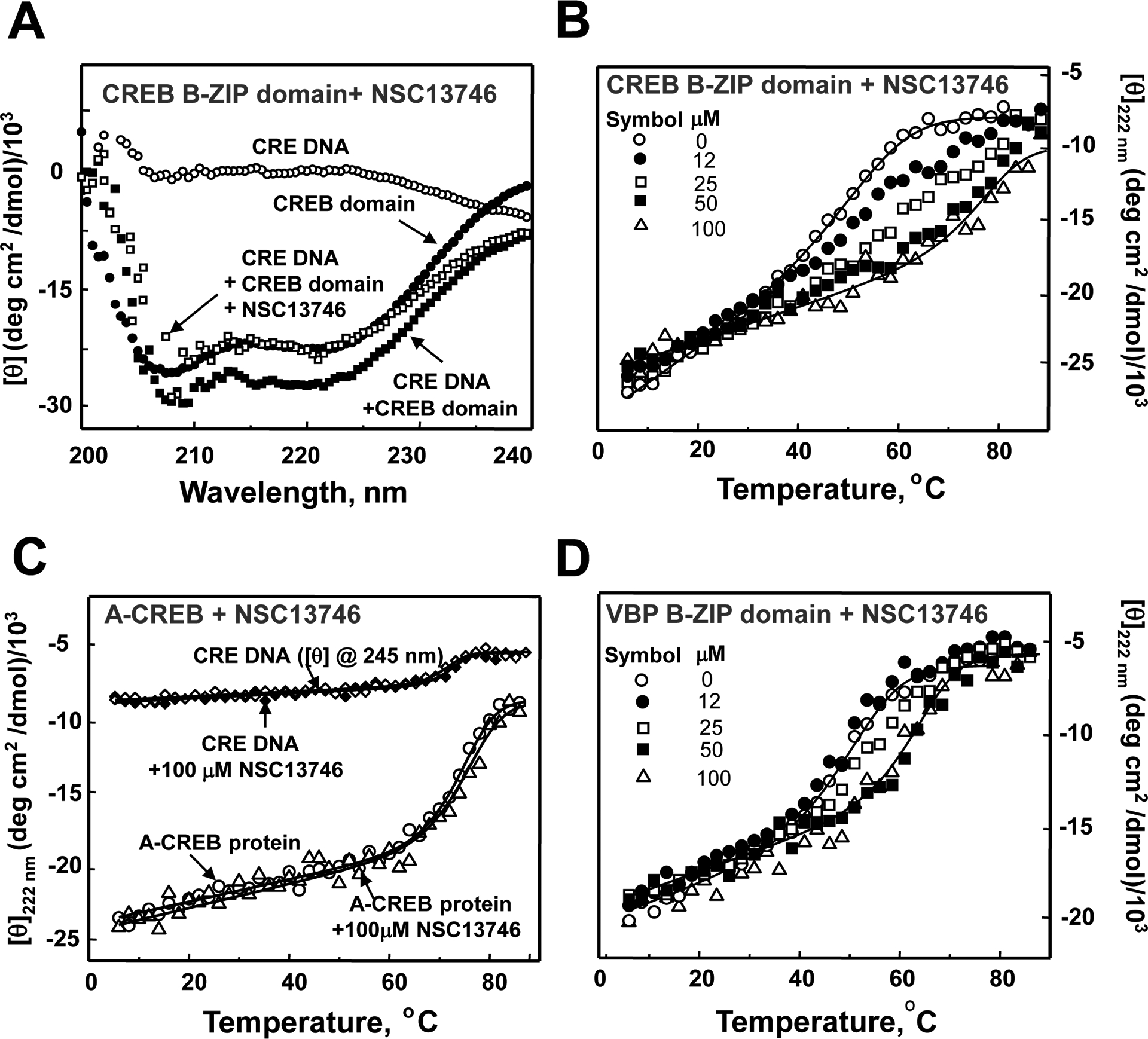
NSC13746 binds to the basic DNA binding domain of CREB. A) CD characterization of NSC13746 interaction with CREB B-ZIP domain. CD spectrum of 2 μM 28 bp double stranded DNA containing a consensus CRE sequence (○), 2 μM of the CREB B-ZIP domain dimer (●), mixture of 2 μM CRE DNA and 2 μM CREB B-ZIP domain dimer (■), mixture of 2 μM CRE DNA, 2 μM CREB B-ZIP domain dimer and 100 μM of NSC13746 heated to 50 °C and cooled to 6 °C (□). B) Thermal stability monitored by CD profiles at 222 nm of CREB B-ZIP domains (2 μM dimer) in the presence of four concentrations of NSC13746 absence (○) and presence of 12 μM (●), 25 μM (□), 50 μM (■), and 100 μM (Δ) of NSC13746. C) CD thermal denaturation studies at 245 nm of 2 μM 28 bp DNA containing unique CRE site and A-CREB protein (222 nm) in absence and presence of NSC13746. A-CREB is a dominant negative form of CREB in which basic DNA binding domain of CREB is replaced by a designed acidic extension such that its homodimer or heterodimer can not bind DNA. Thermal stability of CRE DNA (in absence (◊) and presence (♦)) and A-CREB protein (absence (○) and presence (Δ)) of NSC13746 remains unchanged. D) Thermal denaturation studies of VBP B-ZIP domain in presence of 0–100 μM NSC13746.
