Abstract
Infection with SARS-CoV-2, the cause of coronavirus infectious disease-19 (COVID-19), has caused a pandemic. Few data are available about the risk of COVID-19 infection in persons with hematological cancer, but controversy whether these persons have the same clinical signs and outcomes. We describe a case of life‐threatening COVID-19 infection complicated by severe anemia in patients affected also by chronic myelogenous leukemia. The screening for RBC antibodies and the direct antiglobulin test (DAT) turned positive. The identification of the antibodies, showed the presence of an alloantibody with anti-Lewis b specificity, which was reactive at room temperature, in the anti-human globulin phase (AGH) and with papain-treated red blood cells. At the same time hemophagocytic lymphohistiocytosis (HLH), on the basis of major laboratory findings including hyperferritnemia, increase of triglicerides levels and according to the HLH score was suspected. Patients received antiviral therapy, steroids and intravenous immunoglobulins. Hemolysis resolved and ferritin dramatically decreased after administration of Ig and a Afull recovery was achieved after viral infection resolution.This case highlights the novel and multifaceted hematological findings during sever COVID 19 infection. COVID 19-related pneumonia is mediated by hyper activation of effector T cells and excessive production of inflammatory cytokines, such as IL-6, IL-1, interferon-gamma, and TNF. This inflammatory process called "cytokine storm" is a life-threatening complication of COVID 19 infection. In this case severe immunohematological consequences are reported for the first time and recognition of this complications are probably underestimated.
Keywords: Cml, covid-19, Hemophagocytic lymphohistiocytosis, Cold agglutinin
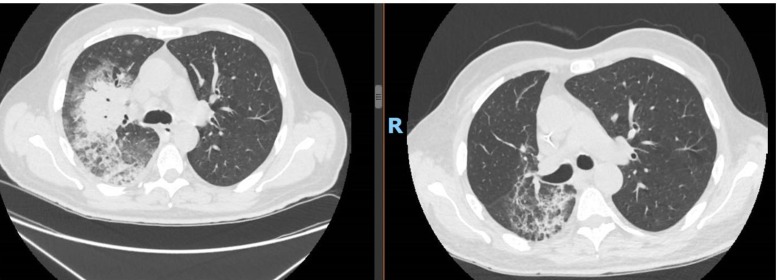
On March 15th, a 55 year patient with chronic myeloid leukemia (CML) was admitted in our Emergency Department (ED). He developed sore throat and fever 38 °C, productive cough and dyspnoea, 5 days before the admission. Few days before admission he received 2 RCB units. He did not have any direct exposure to Coronavirus Sars-Cov2. He had a history of polyarthritis and ulcerative colitis treated by salazopyrin and CML in Imatinib 400 mg. At that time quantitative real-time BCR-ABL was: 0.0034 % consistent with MR 4. On ED the most important findings were: haemoglobin (Hgb) 6.6 g/dl, WBC 19.97* 10^9, neutrophils 18.29*10^9, lymphocyte 0.60 *10^9, basophils 0.60*10^9, platelet counts 136*10^9 / L, high-sensitive C reactive-protein 117.4 mg/L, procalcitonine 0.16 ng/mL, LDH 900 UI/L, pH 4.475, pCO2 27.7 mmHg, pO2 64.8 mmHg, Bicarbonates 20mEq/L, SatO2 95 % on room air. Blood coagulation test revealed international normalized ratio (INR) 1.19 and normal fibrinogen but high D-dimer 35,200 ng/mL. Reticulocytes count was low and haptoglobin was within the normal range. No monoclonal spike was found on electrophoresis. RT-PCR was positive for SARS COV2 on nasopharyngeal swab Computerized tomography (CT) of the chest showed the presence of monolateral lung involvement with “ground glass opacities” and crazy paving appearance (Fig. 1 ).
Fig. 1.
Rx and CT at the diagnosis. “ground glass opacities and crazy paving apparence”.
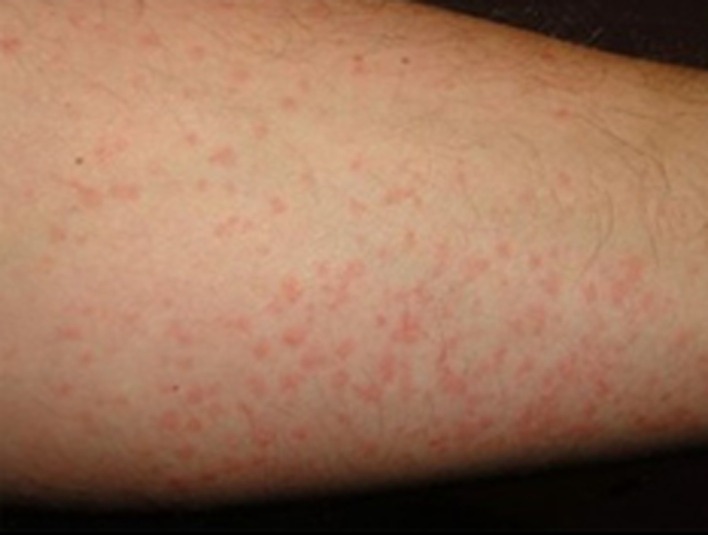
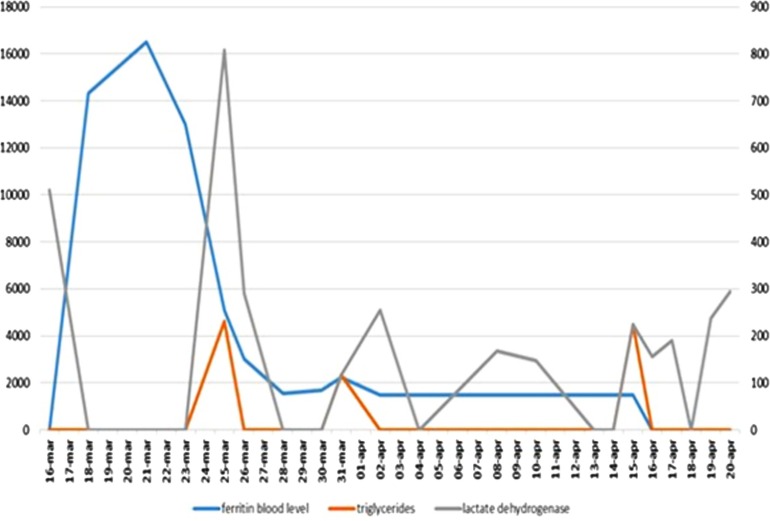
He was treated with low molecular weight heparin, hydroxychloroquine plus lopinavir/ritonavir + ceftriaxone and azithromycin. Blood culture tested negative. On 22nd of March lopinavir was discontinued and darunavir 800 mg/day was added. On 24th of March, during the admission, persisting anemia was noticed and at that time the presence of cold agglutinins was detected. The screening for RBC antibodies and the direct antiglobulin test (DAT) turned positive. DAT investigation with monospecific reagents revealed the presence of IgG, IgM, IgA and C3d (DC Screening, Bio-Rad, Switzerland). The identification of the antibodies, performed using column agglutination technology with commercial red blood cell panels (Identisera and Identisera P, Grifols, Spain), showed the presence of an alloantibody with anti-Lewis b specificity, which was reactive at room temperature, in the anti-human globulin phase (AGH) and with papain-treated red blood cells. Concomitant skin rash developed (Fig. 2 ) and methylprednisolone 20 mg/m2 q12 was started. At the same time hemophagocytic lymphohistiocytosis (HLH), already reported during SARS-COV2 infection [1], on the basis of major laboratory findings including hyperferritinemia, increase of triglicerides levels and according to the HLH score [2], was suspected in our case and intravenous immunoglobulins (IVIG) 20 g/day were administered for 2 consecutive days (Fig. 3 ). The patient received seven RBC units compatible tested at 37 °C, infused using in-line blood warmer without presenting acute or late hemolytic reactions. Four weeks later, antibody screening and identification performed at room temperature, in AGH and with enzyme-treated red cell (Identisera and Identisera P, Grifols, Spain), were completely negative. DAT turned weakly positive (score of agglutination 0.5 +) and only for IgG component (DC Screening, Bio-Rad, Switzerland). Only cold agglutinins at very low title and reactive just at 4 °C were detected. Reticulocytes crisis was observed shortly after IVIG. In this particular case several mechanism seem to be elicited from SARS-COV2 infection giving origin to multifaceted hematological findings. Potential immune-mediated injury in sever COVID 19 seems to be quite frequent and appropriate immunosuppressive treatment particularly in prone subjects can be considered.COVID 19-related pneumonia is mediated by hyper activation of effector T cells and excessive production of inflammatory cytokines, such as IL-6, IL-1, interferon-gamma, and TNF. This inflammatory process may cause a pathological process that leads to plasma leakage, vascular permeability, and disseminated intravascular coagulation; this reaction, called “cytokine storm” is a life-threatening complication of COVID 19 infection. In this case severe immunohematological consequences during SARS-COV-2 are reported and recognition of this complications are probably underestimated, as a matter of fact in May 2020, 9 cases of AIHA and SARS-COV2 infection were published [[3], [4], [5]] and only 3 of them showed cold antibodies mainly in the context of a lymphoproliferative disorder [6] Considering the unusual frequency of chilblainses in children with SARS-COV2 infectionit wi5]l be interesting to study the presence of cold agglutinins and cryoproteins in this particular setting to address if SARS-COV2 may be included in the list of viral agents causing cold agglutinins.
Fig. 2.
Skin rash. Appearance of not-itchy rash to the limbs.
Fig. 3.
Laboratory findings for HLH (Hemophagocytic lymphohistiocytosis).
CRediT authorship contribution statement
Federica Sorà: Conceptualization, Writing - original draft. Patrizia Chiusolo: Writing - original draft. Luca Laurenti: Visualization. Idanna Innocenti: Visualization. Francesco Autore: Data curation. Eleonora Alma: Data curation. Marcello Viscovo: Writing - original draft. Domernico Fusco: Resources. Maddalena Maresca: Resources. Mario Tumbarello: Validation. Simona Sica: Conceptualization.
Declaration of Competing Interest
All the authors declare no conflict of interest.
References
- 1.Mehta P., McAuley D. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;10229:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fardet L., Galicier L. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;9:2613–2620. doi: 10.1002/art.38690. [DOI] [PubMed] [Google Scholar]
- 3.Li M., Nguyen C.B. Evans syndrome in a patient with COVID-19 [published online ahead of print, 2020 May 18] Br J Haematol. 2020 doi: 10.1111/bjh.16846. doi:10.1111/bjh.16846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez C., Kim J. Simultaneous onset of COVID-19 and autoimmune haemolytic anaemia [published online ahead of print, 2020 May 5] Br J Haematol. 2020 doi: 10.1111/bjh.16786. doi:10.1111/bjh.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazarian G., Quinquenel A. Autoimmune hemolytic anemia associated with Covid-19 infection [published online ahead of print, 2020 May 6] Br J Haematol. 2020 doi: 10.1111/bjh.16794. doi:10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randen U., Trøen G., Tierens A. Primary cold agglutinin-associated lymphoproliferative disease: a B-cell lymphoma of the bone marrow distinct from lymphoplasmacytic lymphoma. Haematologica. 2014;99(3):497–504. doi: 10.3324/haematol.2013.091702. [DOI] [PMC free article] [PubMed] [Google Scholar]