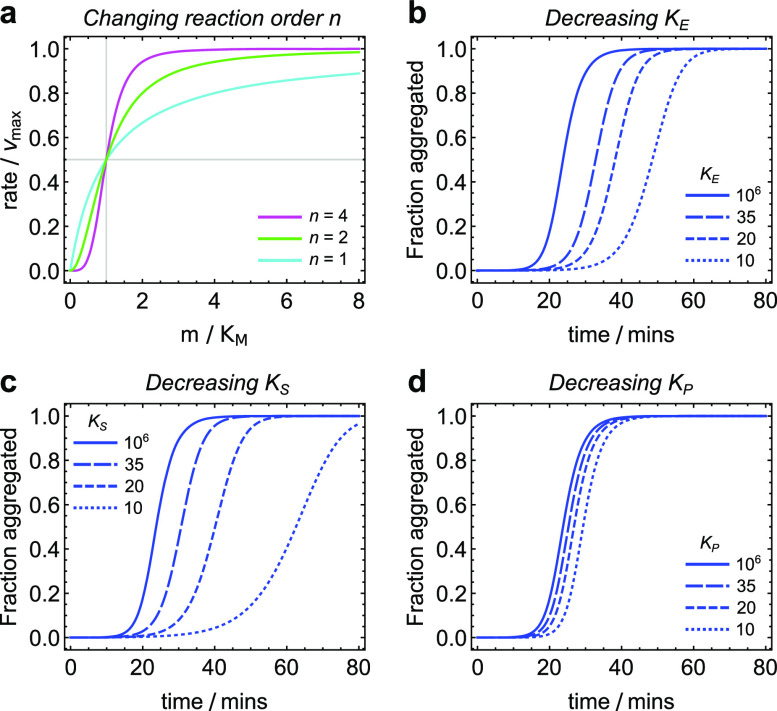
FIG. 2.
(a) Plot of the rate, scaled by the maximal rate , vs the monomer concentration m, scaled by the half-saturation concentration KM, for an elongation reaction (cyan) and for nucleation reactions with orders 2 (green) and 4 (magenta). Elongation obeys Michaelis-Menten kinetics precisely, with a sublinear dependence of the rate on monomer concentration, whereas the higher-order nucleation reactions obey Hill kinetics with the rate exhibiting a sigmoidal monomer dependence. (b)–(d) investigating the effect of saturation in elongation, secondary nucleation, and primary nucleation, respectively, on aggregation curves. Aβ40 rate constants employed with m(0) = 35 µM. Solid lines: KM = 1M, i.e., no saturation. Dashed lines: KM = 35, 20, or 10 µM. Shorter dashed lines correspond to lower saturation concentrations. Saturation in elongation and secondary nucleation mainly reduces the aggregation rate, whereas the sole effect of saturation in primary nucleation is to increase the lag time. Due to the logarithmic dependence of the half time on primary nucleation,26 saturation in the latter has the smallest effect on aggregation kinetics. Saturation in secondary nucleation has the largest overall impact, despite increasing ε, due to the higher reaction order of secondary nucleation compared to elongation.