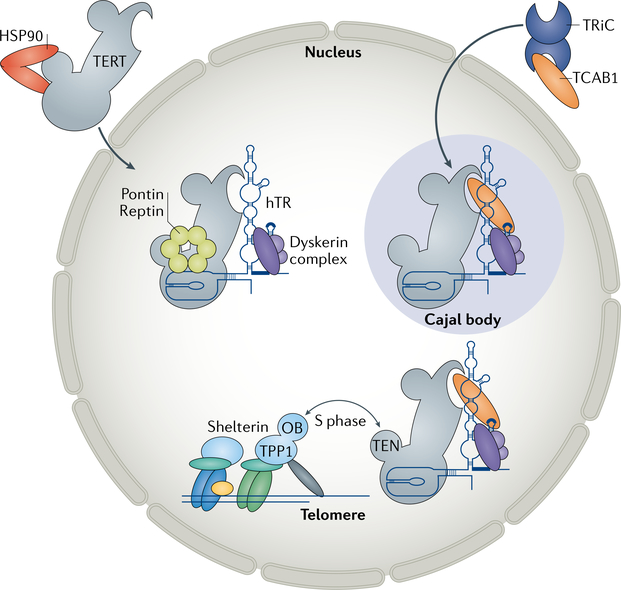
Fig. 3 |. Assembly and trafficking of telomerase within the cell.
Telomerase reverse transcriptase (TERT) is folded with the help of the chaperone heat shock protein 90 (HSP90), whereas human telomerase RNA (hTR) is bound co-transcriptionally by the dyskerin complex, comprising dyskerin, NOP10, NHP2 and the associated factor NAF1. The cofactors pontin and reptin promote assembly of hTR with the dyskerin complex and TERT. Telomerase is localized to Cajal bodies by telomerase Cajal body protein 1 (TCAB1), which is folded by the chaperone TRiC complex. In the absence of TCAB1, telomerase mislocalizes to the nucleolus (not shown) and is not recruited to telomeres. During S phase, the N-terminal domain (TEN) of TERT and the TEL patch (not shown) on the oligosaccharide/oligonucleotide binding (OB) fold domain of TPP1 interact to recruit telomerase to telomeres.