Abstract
Peripheral artery disease (PAD) stems from atherosclerosis of lower extremity arteries with resultant arterial narrowing or occlusion. The most severe form of PAD is termed chronic limb threatening ischemia and carries a significant risk of limb loss and cardiovascular mortality. Diabetes mellitus is known to increase the incidence of PAD, accelerate disease progression, and increase disease severity. Patients with concomitant diabetes and PAD are at high risk for major complications, such as amputation. Despite a decrease in the overall number of amputations performed annually in the United States, amputation rates among those with both diabetes and PAD have remained stable or even increased in high-risk subgroups. Within this cohort, there is significant regional, racial/ethnic, and socioeconomic variation in amputation risk. Specifically, residents of rural areas, Afro-American and Native American patients, and those of low socioeconomic status carry the highest risk of amputation. The burden of amputation is severe, with 5-year mortality rates exceeding those of many malignancies. Furthermore, caring for patients with PAD and diabetes imposes a significant cost to the healthcare system – estimated to range from $84 billion to $380 billion annually. Efforts to improve the quality of care for those with PAD and diabetes must focus on the subgroups at high-risk for amputation and the disparities they face in the receipt of both preventive and interventional cardiovascular care. Better understanding of these social, economic, and structural barriers will prove to be crucial for cardiovascular physicians striving to better care for patients facing this challenging combination of chronic diseases.
Keywords: Diabetes, Peripheral artery disease, Amputation, Epidemiology
Subject Codes: Diabetes, Type 1; Diabetes, Type 2; Risk Factors; Atherosclerosis; Peripheral Vascular Disease; Vascular Disease; Health Services; Quality and Outcomes
Graphical Abstract
Introduction
Peripheral artery disease (PAD) is the development of chronic arterial occlusive disease of the lower extremities due to atherosclerosis.1 PAD is associated with atherosclerosis of other vascular beds, and the presence of diabetes mellitus is known to both increase the incidence of PAD, as well as accelerate disease progression and worsen disease severity.2 Given this, patients with concomitant diabetes and PAD are at high risk for ischemic events and subsequent amputation.3 Further, within this cohort, there is significant regional, racial and ethnic, and socioeconomic variation in the risk of amputation.3–5 Specifically, residents of rural areas, Afro-American and Native American patients, and those of low socioeconomic status carry the highest risk of amputation. The purpose of this brief review, therefore, is to describe the epidemiology of diabetes and PAD in the United States (US), outline the risk of amputation in the high-risk cohort of patients with concomitant diabetes and PAD, and describe the variation in this risk seen across socioeconomic and racial and ethnic strata.
Epidemiology of diabetes mellitus in the United States
Diabetes mellitus is a disorder stemming from glucose dysregulation. Of those persons with diabetes, 90% to 95% have type 2 diabetes while 5% to 10% have type 1 diabetes.6 Within the United States, more than 30 million Americans have diabetes and an additional 84 million Americans meet diagnostic criteria for pre-diabetes.7 This represents greater than a 200% increase in disease prevalence over the preceding two decades (Figure 1).7 Meanwhile, the number of incident cases of diabetes in US adults has decreased each year since 2008 (Figure 1).8
Figure 1:
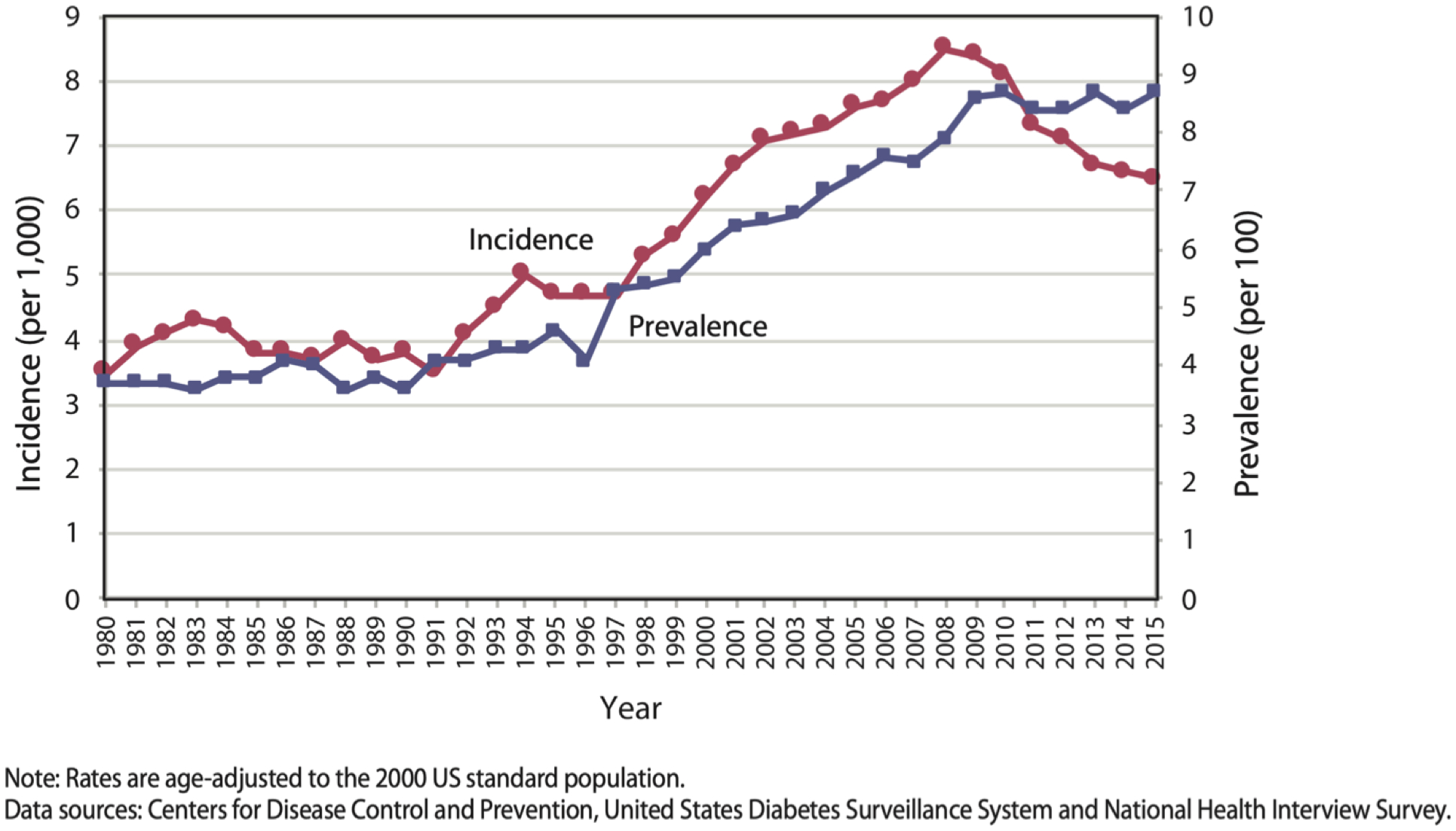
Trends in Incidence and Prevalence of Diagnosed Diabetes Among Adults Aged 18 or Older, United States, 1980–2015.8
While type 2 diabetes has historically been a disease diagnosed in adults over the age 40, between 2001 and 2009 there was a 30.5% increase in the overall prevalence of type 2 diabetes among people less than 20 years old.9 In this study, subgroup analysis demonstrated significant increases in the prevalence of diabetes in both sexes, those ages 10–14, and those ages 15–19 across the study period. When stratified by race and ethnicity, significant increases in prevalence were seen in White, Afro-American, and Hispanic youth, but non-significant changes in prevalence rates were seen in Asian Pacific Islander and Native American youth. This increase in disease burden among American youth carries important implications regarding the increased risk of early diabetic complications as this cohort transitions into adulthood.9
Race, ethnicity, regions, and differences in diabetes
There is significant variation in the prevalence of diabetes across races and ethnicities in the US. The lowest prevalence is seen among White adults, where the prevalence of diabetes is estimated at 7.3% for females and 9.4% for males. Conversely, the rates among Afro-American adults are estimated at 14.7% and 13.4%, among Hispanic adults 15.1% and 14.1%, and among Asian adults 12.8% and 9.9% for males and females, respectively. The highest prevalence of diabetes is seen among Native American and Alaska Native adults, with a prevalence of 14.9% and 15.3% for males and females respectively.6, 10
A report issued by the Centers for Disease Control in 2016 outlined regional geographic variation in diabetes prevalence across the US. The prevalence of total diabetes across counties within the US ranged from 1.5% to 33.0% in 2016 according to the Centers for Disease Control.10 The counties with the highest prevalence were primarily located in the South, along the US-Mexican border, and in the Midwestern region.8 This variation and distribution is consistent with prior work documenting trends in US regional variation of diabetes prevalence between 1999 and 2012.11
Diabetes is a significant risk factor for atherosclerosis within all vascular beds, including PAD, but is also associated with overall cardiovascular disease morbidity and mortality.2 Cardiovascular disease associated death rates have been shown to be significantly higher for diabetic patients, largely due to the increased risk of stroke and myocardial infarction, compared to those without diabetes.10, 12–14 The magnitude of the impact of diabetes on cardiovascular death varies across studies and models from an 18% increase to a four-fold increase in mortality8, 14. Following a myocardial infarction, diabetic patients have higher rates of morbidity, mortality, and repeat infarction than non-diabetic patients.15–19 A recent systematic literature review found that cardiovascular disease affects 32.2% of patients with type 2 diabetes and accounts for 50.3% of all deaths in patients with type 2 diabetes.20
Epidemiology of Peripheral Artery Disease in the United States
Peripheral artery disease is defined as chronic arterial occlusive disease of the lower extremities and varies in severity. While many individuals with PAD are asymptomatic, intermittent claudication is the typical presenting symptom. This is characterized by burning, aching, cramping, fatigue, or numbness in the calves, thighs, or buttocks with ambulation or exercise and is quickly and completely relieved by rest. The most severe manifestation of PAD is termed chronic limb threatening ischemia (CLTI) and is typified by rest pain or ischemic ulceration or dry gangrene.21 Ischemic rest pain stems from chronic sensory nerve ischemia and presents as a diffuse burning or aching pain in the forefoot.1 In many, this pain is worsened with elevation of the extremity and somewhat improved with dependent positioning of the limb. Tissue loss is the development of ulceration, gangrene, or infection in the foot or leg that may ultimately lead to amputation.
Challenges in measuring the prevalence of PAD
Accurate epidemiologic information on PAD is limited by the fact that only approximately 10% of patients with PAD demonstrate the typical symptomology of intermittent claudication.22, 23 This results in a large population with preclinical disease or uncharacteristic symptoms that may not be accounted for. Given this, prevalence estimates have relied on community screening or data on symptomatic patients. Through these approaches, it is estimated that PAD affects between 8.5 and 12 million Americans.4, 24 This prevalence has increased by an estimated 25% over the preceding decade. In high-income countries, such as the US, prevalence increased by 13.1% between 2000 and 2010. In low and middle-income countries, prevalence increased by 28.7% over the same time course.25
Prevalence of PAD increases significantly with age, such that by age 80 prevalence is over 20% compared with <5% in those less than 50 years of age.26 There is variation in prevalence of PAD across races and ethnicities with Afro-American patients affected more frequently.3, 4 Specifically, the prevalence of PAD among Afro-American patients is twice that of non-Hispanic White patients in all age groups. The prevalence of PAD among Hispanics, Asian Americans, and Native Americans is similar to that of non-Hispanic White patients. Investigation into the variation seen across racial and ethnic groupings has demonstrated confounding by education and other socioeconomic variables.27 This does not discount the variation seen across ethnicities or races but highlights the impact of socioeconomic factors on the prevalence of PAD.
Risk factors impacting PAD prevalence and progression
In addition to age, significant atherosclerotic risk factors for PAD include cigarette smoking, dyslipidemia, and diabetes. Cigarette smoking is estimated to double the risk of PAD, though some studies have found smoking to increase the risk nearly fourfold.28, 29 Dyslipidemia is estimated to nearly double the risk of PAD.30 Similarly, diabetes has been associated with a two to fourfold increase in the prevalence of PAD.2 Further, smoking, diabetes, and dyslipidemia have been associated with progression of PAD and worsening lower extremity arterial perfusion.26, 30
Chronic Limb Threatening Ischemia
CLTI, the most severe form of PAD, develops in approximately 11% of patients with PAD.1, 31 Population studies estimate that the prevalence of CLTI in the US adult population over the age of 40 is 1.28%, which totals approximately 2 million individuals.32 CLTI carries both an elevated risk of amputation and an elevated risk of cardiovascular morbidity and mortality that varies with CLTI severity. A recent systematic review noted 1-year amputation rates ranged from 15–20% and 1-year mortality rates ranged from 15–40%.33 The significant variation in amputation and mortality rates can be attributed, in part, to the variation in definitions of CLTI used across studies. To more precisely evaluate the natural history of CLTI, a recent meta-analysis evaluated studies which included only patients with untreated, severe CLTI and found a 1-year major amputation rate of 22% and a 1-year mortality of 22%.34
Concomitant Diabetes and PAD
Diabetes and PAD independently carry a risk of amputation.1 The pathophysiology of each is important to understanding the natural history of each disease process and have been the subject of recent reviews.35, 36 Determination of the epidemiology of concomitant diabetes and PAD faces similar challenges as measurement of disease prevalence in PAD alone. While the diagnosis of diabetes is well described, there is broad variability in the severity and symptomology of PAD.37 Thus, many patients may remain asymptomatic with preclinical disease. As a result, the true prevalence of concomitant PAD and diabetes is difficult to ascertain. Using best estimates, diabetes has been associated with a two to fourfold increase in the prevalence of PAD.2 Of those with PAD, approximately 20–30% have concomitant diabetes.38 Not only does diabetes result in an increase in the incidence of PAD, but it also increases the severity of the disease state.39 Specifically, patients with diabetes and PAD more frequently have infrapopliteal or tibial artery disease and vessel calcification compared with non-diabetic patients.39, 40
Risk of amputation in patients with Diabetes and PAD
Nearly 100,000 major leg amputations are performed every year in the US. Of these, over half are attributable to diabetes and PAD.41, 42 Within the population of PAD patients with CLTI, the estimated prevalence of diabetes ranges from 27% to 76%.43 This cohort, with concomitant diabetes and PAD, carries a risk of amputation that is four times higher than the national average.41, 42, 44, 45 Studies have demonstrated that 25% to 90% of amputations within studied populations are associated with diabetes.46, 47 This risk is thought to be attributable to the combination of peripheral neuropathy and infection stemming from diabetes and the presence of impaired arterial flow due to PAD. Diabetic ulcers, associated with motor, sensory, and autonomic neuropathy, resultant foot deformities, and impaired wound healing, also are integral to the risk of amputation in patients with diabetes. Of those with a diabetic ulcer, though, it is estimated that approximately 50% have concomitant PAD.48
Trends in overall amputation risk over time
Despite the increase in the burden of diabetic disease, the overall rate of major amputations in the US has decreased.41, 42, 44, 49 Specifically, rates of major lower extremity amputation decreased by 40% between 1996 and 2011 among US Medicare patients.42, 50 This resulted in a major amputation rate of 188 per 100,000 Medicare patients (Figure 2). During this same time period, a shift is observed in revascularization approaches (Figure 2). A 71% decline in the rate of open surgical bypass was observed (201 to 83 per 100,000) along with an incommensurate increase in the rate of endovascular interventions (138 to 584 per 100,000).42 Given the multifaceted and complex natural history of CLTI, it cannot be concluded that the decrease in amputation rates is solely attributable to the shift in revascularization approaches and intensity.42, 50 Concomitant advancements in medical and wound care therapies along with the establishment of integrated wound care centers have impacted the care of patients with CLTI and, therefore, may have also assisted to decrease the overall rate of amputation.42, 51
Figure 2:
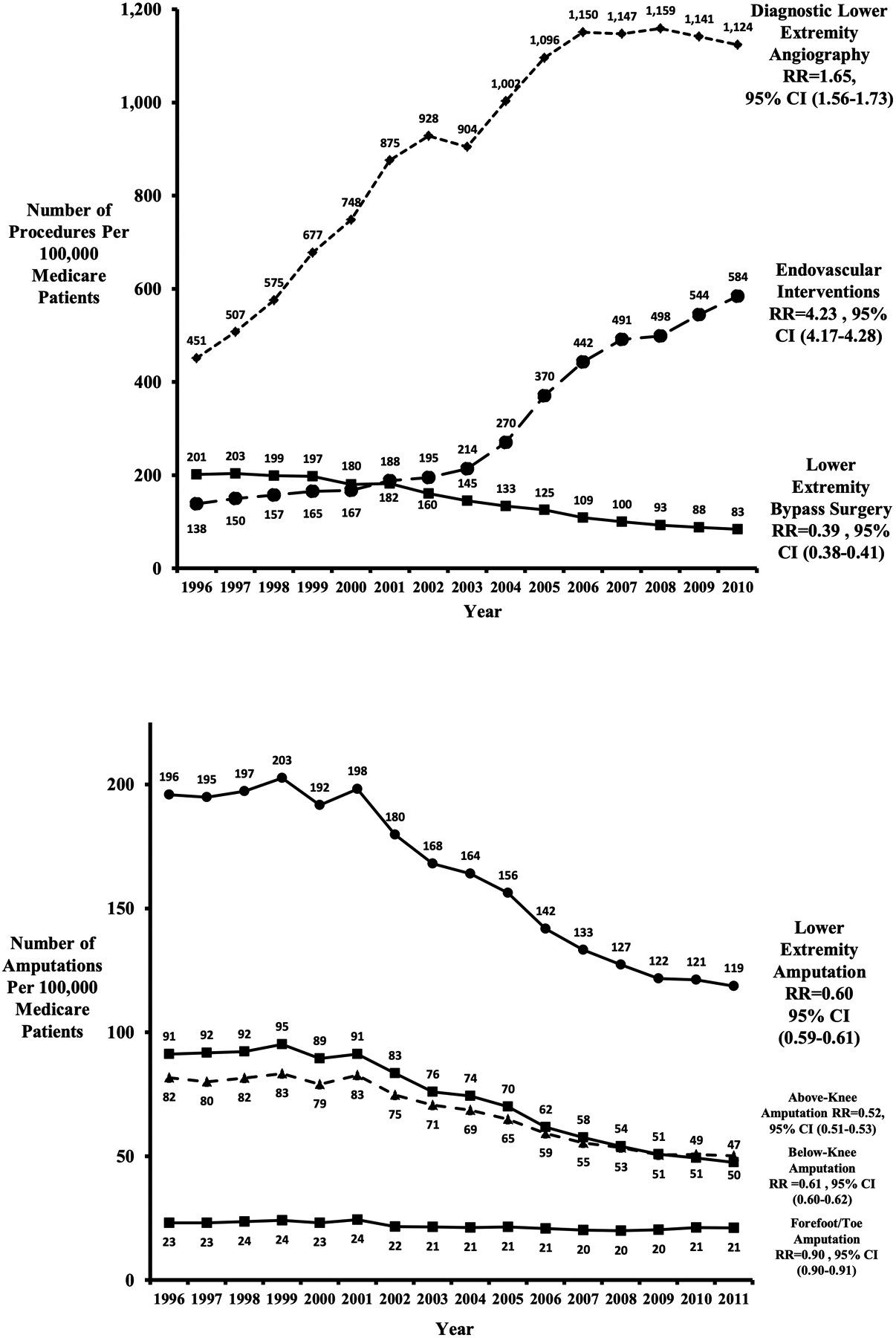
Trends in endovascular interventions, major amputation, and lower extremity bypass surgery 1996–2011. RR, Risk ratio; CI, confidence interval.50
Trends in amputation risk among those with PAD and diabetes over time
Despite the overall decline in amputation rates described above, the trends in amputation among those with concomitant PAD and diabetes differ. A study within the state of California found amputation rates in this cohort had declined between 2005 and 2008 but had plateaued and remained stable through 2011 at approximately 31 per 1 million Californians.52 An additional study from the same group focused on those with ulceration found amputation rates increased nearly three-fold between 2005 and 2013, from 10% to nearly 30%, among patients in California with diabetes and CLTI.45 These data demonstrate that risks for patients with diabetes and PAD remain a significant priority in amputation prevention, even as results have improved for other patient subgroups.
Disparities in amputation risk
Within the United States, disparities in race, ethnicity, and geography exist such that amputation rates can be up to nine times higher – 1,800 per 100,000 Medicare patients - among Afro-Americans with diabetes in certain rural regions compared with non-Afro-Americans in more urban regions.3, 42 Henry et al. demonstrated an increased odds of amputation among Afro-American (OR 2.9, p<0.0001) and Native American (OR 2.4, p<0.0001) patients with PAD compared to White patients with PAD.5 Similarly, Goldberg et al. demonstrated that Afro-American patients with diabetes have an increased risk of amputation when compared with White patients with diabetes across risk strata.41 Within this study, 25.1% of the amputee cohort was Afro-American compared with 12.6% of the non-amputee cohort. Further, Rizzo et al. demonstrated Native American patients with PAD were twice as likely to undergo amputation than non-Hispanic White patients with PAD.53
Evaluation of revascularization attempts prior to amputation demonstrated that Afro-American patients were significantly less likely to undergo revascularization (Figure 3) when compared with White patients who had undergone amputation (OR 0.72).54 This same work similarly showed Afro-American patients who had undergone amputation were less likely to have been admitted or undergone wound debridement compared with White patients. The timing of revascularization in relation to amputation was similar across races/ethnicities and across varying durations of time, thus suggesting that late presentation could not explain the differences in care.54
Figure 3:
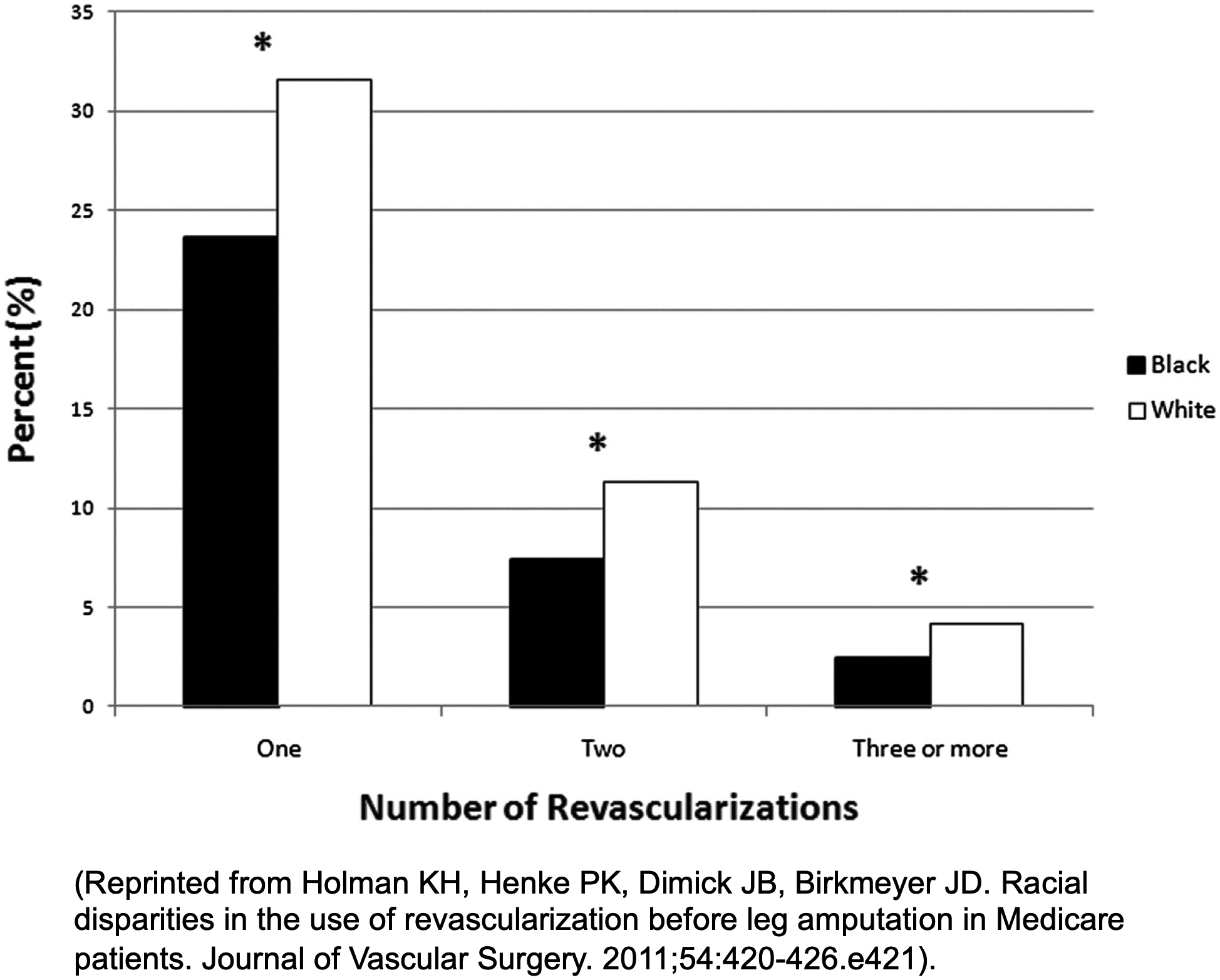
Percentage of patients undergoing revascularization prior to amputation. *P < .0001.54
In terms of socioeconomic status, PAD patients with lower socioeconomic status and Medicaid insurance coverage are more likely to undergo amputation.5, 55 Specifically, those in the lowest income quartile were at 34% higher odds of undergoing an amputation when compared to those in the highest income quartile (OR 1.34, p<0.0001).5 Similarly, Medicaid coverage status placed patients at higher odds of amputation when compared to those with Medicare coverage (OR 1.26, p<0.0001).
Broad variation has also been demonstrated at the international level where mean incidence rates of major amputations across different European and Australasian nations varied nearly 6-fold.56 Within this analysis, factors such as the prevalence of diabetes or smoking, population age, and healthcare reimbursement models, such as fee-for-service versus population-based reimbursement, were compared. While each varied, only healthcare reimbursement models yielded a significant difference in amputation rates. Major amputations were less common in fee-for-service nations compared to nations with population-based reimbursement models. Similarly, amputations were more common in countries with lower national gross domestic product per capita and healthcare expenditures as a percent of gross domestic product.
Burden and cost of amputation
Amputation related to diabetes and PAD presents a tremendous burden on patients, families, and society. At the patient level, of those who undergo amputation as a result of diabetes and/or PAD, more than 55% are permanently disabled thereafter. A similar number, especially those who undergo above knee amputation, never return to an ambulatory status.57 Among patients who had undergone major amputation for CLTI (amputation either below or above the knee), 1-year mortality was 40.4% in a recent US Medicare study; this was 10 percent higher, in absolute terms, when compared to patients diagnosed with CLTI who did not undergo amputation, where the 1-year mortality rate was 30%.32, 59 These rates manifest the severity of this disease over time, as the 5-year mortality associated with CLTI without amputation is estimated to be 55% to 65%.32 As shown in Table 1, this exceeds the 5-year mortality of female breast cancer (10%), colon cancer (35%), and myeloma (50%).58
Table 1.
Comparison of 5-Year Mortality Rates Across Disease Processes
Estimates of financial impact of amputation
Within health systems, patients with concomitant diabetes and PAD pose a significant strain on the United States’ healthcare system. Total annual Medicare expenditures on these patients with PAD are estimated to exceed $84 billion per year, with some estimates ranging to as high as $381 billion per year if costs associated with long term care are included.59–61 Among individuals with PAD who require intervention, those who also have diabetes are the costliest with an average annual Medicare spending of approximately $120,000 per patient compared with $70,000 in those without diabetes per treatment year.60
Addressing amputation risk in patients with diabetes and PAD
For patients with diabetes and PAD, preventative care and care coordination are critical in avoiding progression to CLTI, ulceration, and eventually amputation. Integrated management strategies for diabetes and CLTI, defined as hemoglobin A1c testing, diabetic foot care, and vascular assessment, have been shown to help limit amputation in patients with diabetes and CLTI.62–64 Furthermore, each of these tests or examinations are routine, frequently ordered, and inexpensive.62 These integrated management strategies have been put forward by the American Heart Association, the National Committee on Quality Assurance, the American Diabetes Association, and the Society for Vascular Surgery, among others, in the form of practice guidelines outlining evidence-based benchmarks of care for patients with PAD and recommendations for risk factor modification (Table 2).65–68
Table 2.
Integrated management strategies to limit amputation for patients with diabetes and CLTI.
| Integrated management for diabetes and CLTI | ||||
|---|---|---|---|---|
| Organization | Program / Document | Hemoglobin A1c testing | Diabetic Foot Care | Vascular Assessment |
| HRSA70, 71 | LEAP (Lower Extremity Amputation Prevention Program) | HgA1c, patient education | Annually | Physical Exam and ABI |
| AHA/APMA/SVS68 | Strategies to Prevent and Heal Diabetic Foot Ulcers | HgA1c, patient education | Annually | Physical Exam and ABI |
| VAMC72–74 | STAMP (Special Teams for Amputation, Mobility, and Prosthetics | HgA1c, patient education | Annually | Physical Exam and ABI |
| Healthy People 202075 | Diabetic Objective D-4 | HgA1c, patient education | Annually | Physical Exam and ABI |
| ADA66 | Position Statement on Preventive Foot Care | HgA1c, patient education | Annually | Physical Exam and ABI |
| International Diabetes Federation76 | Position Statement on Preventive Foot Care | HgA1c, patient education | Annually | Physical Exam and ABI |
Despite these recommendations, implementation of these guidelines in practice varies. As it pertains to hemoglobin A1c testing, specifically, a report from The Dartmouth Atlas in 2014 demonstrated this variation in utilization using Medicare claims data (Figure 4).69 Hemoglobin A1c testing was utilized relatively less frequently in the Southern and Western regions of the United States (Albuquerque, NM 66.9%, Anchorage, AK 69.8%, Lawton, OK 73.8% percent of diabetic Medicare beneficiaries received testing) when compared to the upper Midwest (Rochester, MN 92.7%, Marshfield, WI 92.3%).69 There was also significant variation in the care provided when stratified by ethnicity. Afro-American patients were less likely to receive the recommended preventative measures. Specifically, 84.2% of non-Afro-American patients received hemoglobin A1C testing, while only 80.9% of Afro-American patients underwent this testing.
Figure 4:
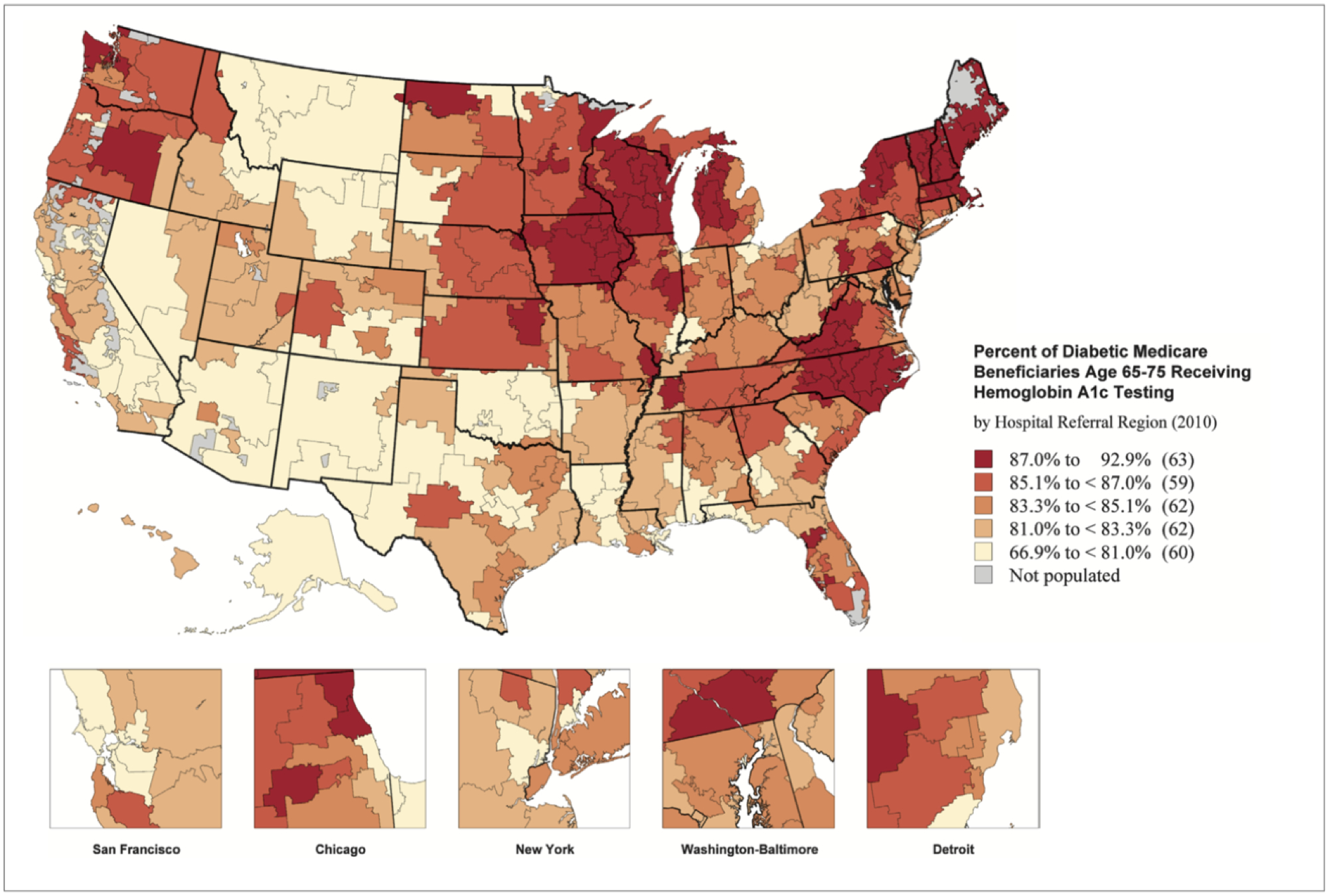
Percent of diabetic Medicare beneficiaries receiving hemoglobin A1c testing (2010). Dartmouth Atlas.69
Conclusion
Overall, the concomitant existence of PAD and diabetes lends significant complexity to the management of these conditions. Patients with both conditions face significantly higher risks of amputation than patients with either disease process in isolation. These conditions, especially in certain populations of patients, impart several-fold higher risks of amputation and poor survival, and these risks are greatest in many of the United States’ most vulnerable populations. Further, while complex and expensive treatments such as endovascular interventions are often necessary, research suggests that simple, inexpensive, and evidence-based measures, such as hemoglobin A1c testing, diabetic foot care, and vascular assessments, are underused.
The pathway ahead for those who care for patients with PAD and diabetes will require a better understanding of which groups within these high-risk subgroups – Native Americans, Afro-Americans, the rural poor – are subject to the highest risks of amputation. A clearer vision of the epidemiology, pathophysiology, and health care delivery present in these populations, as well as the social, economic, and structural barriers which are implicit in their excess risks of limb loss, is urgently needed. While these tasks present obvious challenges, it is important that in further limiting amputation risk, we study the patients at the greatest risk where amputation rates remain unacceptably high. The challenges ahead will be multidisciplinary and complex, but successful reduction of amputation risk among patients with PAD and diabetes is too important of a goal to fade from these challenges.
Highlights.
Concomitant diagnoses of peripheral artery disease (PAD) and diabetes mellitus have a negative synergistic effect, leaving patients at a higher risk for amputation than either of the two diseases alone.
Of those with PAD, patients of low socioeconomic status, those residing in rural areas, and those of Afro-American or Native American ethnicity are at the highest risk of amputation
Over the preceding two decades, the rates of endovascular interventions have risen while inexpensive and evidence-based measures – such as hemoglobin A1c testing – are underused.
Acknowledgments
We appreciate the excellent artistic abilities of Shayne E. Dodge and her willingness to put them towards the graphic abstract.
Sources of funding
This publication was supported by American Heart Association Strategically Focused Research Network Grant (18SFRN33900147) “Understanding the Pathobiology and Predictors of Limb Ischemia to Improve Outcomes in Peripheral Artery Disease and Diabetes”.
Abbreviations
- PAD
peripheral artery disease
- US
United States
- CLTI
chronic limb threatening ischemia
Footnotes
Disclosures
None.
References
- 1.Sidawy AN, Perler BA. Rutherford’s vascular surgery and endovascular therapy. Philadelphia, PA: Elsevier; 2019. [Google Scholar]
- 2.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581 [DOI] [PubMed] [Google Scholar]
- 3.Newhall K, Spangler E, Dzebisashvili N, Goodman DC, Goodney P. Amputation rates for patients with diabetes and peripheral arterial disease: The effects of race and region. Ann Vasc Surg. 2016;30:292–298 e291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic-specific prevalence of peripheral arterial disease in the united states. Am J Prev Med. 2007;32:328–333 [DOI] [PubMed] [Google Scholar]
- 5.Henry AJ, Hevelone ND, Belkin M, Nguyen LL. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia. Journal of Vascular Surgery. 2011;53:330–339.e331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation. 2019;139:e56–e528 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Division of diabetes translation at a glance. 2019;2019 [Google Scholar]
- 8.Centers for Disease Control and Prevention. Diabetes report card 2017. 2018
- 9.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. National diabetes statistics report, 2017. 2017
- 11.Dwyer-Lindgren L, Mackenbach JP, van Lenthe FJ, Flaxman AD, Mokdad AH. Diagnosed and undiagnosed diabetes prevalence by county in the u.S., 1999–2012. Diabetes Care. 2016;39:1556–1562 [DOI] [PubMed] [Google Scholar]
- 12.An Y, Zhang P, Wang J, et al. Cardiovascular and all-cause mortality over a 23-year period among chinese with newly diagnosed diabetes in the da qing igt and diabetes study. Diabetes Care. 2015;38:1365–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor KS, Heneghan CJ, Farmer AJ, Fuller AM, Adler AI, Aronson JK, Stevens RJ. All-cause and cardiovascular mortality in middle-aged people with type 2 diabetes compared with people without diabetes in a large u.K. Primary care database. Diabetes Care. 2013;36:2366–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghavan S, Vassy JL, Ho YL, Song RJ, Gagnon DR, Cho K, Wilson PWF, Phillips LS. Diabetes mellitus–related all‐cause and cardiovascular mortality in a national cohort of adults. Journal of the American Heart Association. 2019;8:e011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The framingham study. JAMA. 1979;241:2035–2038 [DOI] [PubMed] [Google Scholar]
- 16.Fuller JH, Shipley MJ, Rose G, Jarrett RJ, Keen H. Mortality from coronary heart disease and stroke in relation to degree of glycaemia: The whitehall study. Br Med J (Clin Res Ed). 1983;287:867–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haffner SM. Coronary heart disease in patients with diabetes. N Engl J Med. 2000;342:1040–1042 [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB. Framingham study insights on diabetes and cardiovascular disease. Clin Chem. 2011;57:338–339 [DOI] [PubMed] [Google Scholar]
- 19.Matheus AS, Tannus LR, Cobas RA, Palma CC, Negrato CA, Gomes MB. Impact of diabetes on cardiovascular disease: An update. Int J Hypertens. 2013;2013:653789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17:83–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg. 2019;58:S1–S109.e133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324 [DOI] [PubMed] [Google Scholar]
- 23.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, Chan C, Celic L, Pearce WH, Schneider JR, Sharma L, Clark E, Gibson D, Martin GJ. Leg symptoms in peripheral arterial disease: Associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606 [DOI] [PubMed] [Google Scholar]
- 24.Thiruvoipati T, Kielhorn CE, Armstrong EJ. Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World J Diabetes. 2015;6:961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet. 2013;382:1329–1340 [DOI] [PubMed] [Google Scholar]
- 26.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526 [DOI] [PubMed] [Google Scholar]
- 27.Collins TC, Petersen NJ, Suarez-Almazor M, Ashton CM. Ethnicity and peripheral arterial disease. Mayo Clin Proc. 2005;80:48–54 [DOI] [PubMed] [Google Scholar]
- 28.Allison MA, Criqui MH, McClelland RL, Scott JM, McDermott MM, Liu K, Folsom AR, Bertoni AG, Sharrett AR, Homma S, Kori S. The effect of novel cardiovascular risk factors on the ethnic-specific odds for peripheral arterial disease in the multi-ethnic study of atherosclerosis (mesa). J Am Coll Cardiol. 2006;48:1190–1197 [DOI] [PubMed] [Google Scholar]
- 29.Murabito JM, Evans JC, Nieto K, Larson MG, Levy D, Wilson PW. Prevalence and clinical correlates of peripheral arterial disease in the framingham offspring study. Am Heart J. 2002;143:961–965 [DOI] [PubMed] [Google Scholar]
- 30.Kennedy M, Solomon C, Manolio TA, Criqui MH, Newman AB, Polak JF, Burke GL, Enright P, Cushman M. Risk factors for declining ankle-brachial index in men and women 65 years or older: The cardiovascular health study. Arch Intern Med. 2005;165:1896–1902 [DOI] [PubMed] [Google Scholar]
- 31.Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, Zakharyan A, Hirsch AT. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured national population. J Vasc Surg. 2014;60:686–695.e682 [DOI] [PubMed] [Google Scholar]
- 32.Duff S, Mafilios MS, Bhounsule P, Hasegawa JT. The burden of critical limb ischemia: A review of recent literature. Vasc Health Risk Manag. 2019;15:187–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duff S, Mafilios MS, Bhounsule P, Hasegawa JT. The burden of critical limb ischemia: A review of recent literature. Vascular health and risk management. 2019;15:187–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abu Dabrh AM, Steffen MW, Undavalli C, Asi N, Wang Z, Elamin MB, Conte MS, Murad MH. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62:1642–1651 e1643 [DOI] [PubMed] [Google Scholar]
- 35.Noor S, Zubair M, Ahmad J. Diabetic foot ulcer--a review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr. 2015;9:192–199 [DOI] [PubMed] [Google Scholar]
- 36.Morley RL, Sharma A, Horsch AD, Hinchliffe RJ. Peripheral artery disease. Bmj. 2018;360:j5842. [DOI] [PubMed] [Google Scholar]
- 37.Brownrigg JR, Hinchliffe RJ, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, Reekers J, Shearman CP, Zierler RE, Schaper NC. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: A systematic review. Diabetes Metab Res Rev. 2016;32 Suppl 1:128–135 [DOI] [PubMed] [Google Scholar]
- 38.Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47:921–929 [DOI] [PubMed] [Google Scholar]
- 39.Jude EB, Oyibo SO, Chalmers N, Boulton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: A comparison of severity and outcome. Diabetes Care. 2001;24:1433–1437 [DOI] [PubMed] [Google Scholar]
- 40.Sheehan P Peripheral arterial disease in people with diabetes: Consensus statement recommends screening. Clinical Diabetes. 2004;22:179–180 [Google Scholar]
- 41.Goldberg JB, Goodney PP, Cronenwett JL, Baker F. The effect of risk and race on lower extremity amputations among medicare diabetic patients. J Vasc Surg. 2012;56:1663–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54–60 [DOI] [PubMed] [Google Scholar]
- 43.Dick F, Diehm N, Galimanis A, Husmann M, Schmidli J, Baumgartner I. Surgical or endovascular revascularization in patients with critical limb ischemia: Influence of diabetes mellitus on clinical outcome. J Vasc Surg. 2007;45:751–761 [DOI] [PubMed] [Google Scholar]
- 44.Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA. Lower-extremity amputation in people with diabetes. Epidemiology and prevention. Diabetes Care. 1989;12:24–31 [DOI] [PubMed] [Google Scholar]
- 45.Humphries MD, Brunson A, Li CS, Melnikow J, Romano PS. Amputation trends for patients with lower extremity ulcers due to diabetes and peripheral artery disease using statewide data. J Vasc Surg. 2016;64:1747–1755 e1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agarwal S, Sud K, Shishehbor MH. Nationwide trends of hospital admission and outcomes among critical limb ischemia patients: From 2003–2011. J Am Coll Cardiol. 2016;67:1901–1913 [DOI] [PubMed] [Google Scholar]
- 47.Unwin N Epidemiology of lower extremity amputation in centres in europe, north america and east asia. Br J Surg. 2000;87:328–337 [DOI] [PubMed] [Google Scholar]
- 48.Prompers L, Huijberts M, Apelqvist J, et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in europe. Baseline results from the eurodiale study. Diabetologia. 2007;50:18–25 [DOI] [PubMed] [Google Scholar]
- 49.Jones WS, Patel MR, Dai D, Subherwal S, Stafford J, Calhoun S, Peterson ED. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: Results from u.S. Medicare 2000–2008. J Am Coll Cardiol. 2012;60:2230–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodney PP, Tarulli M, Faerber AE, Schanzer A, Zwolak RM. Fifteen-year trends in lower limb amputation, revascularization, and preventive measures among medicare patients. JAMA surgery. 2015;150:84–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flores AM, Mell MW, Dalman RL, Chandra V. Benefit of multidisciplinary wound care center on the volume and outcomes of a vascular surgery practice. J Vasc Surg. 2019 [DOI] [PubMed]
- 52.Humphries MD, Brunson A, Hedayati N, Romano P, Melnkow J. Amputation risk in patients with diabetes mellitus and peripheral artery disease using statewide data. Annals of Vascular Surgery. 2016;30:123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rizzo JA, Chen J, Laurich C, Santos A, Martinsen BJ, Ryan MP, Kotlarz H, Gunnarsson C. Racial disparities in pad-related amputation rates among native americans and non-hispanic whites: An hcup analysis. J Health Care Poor Underserved. 2018;29:782–800 [DOI] [PubMed] [Google Scholar]
- 54.Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in medicare patients. Journal of Vascular Surgery. 2011;54:420–426.e421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eslami MH, Zayaruzny M, Fitzgerald GA. The adverse effects of race, insurance status, and low income on the rate of amputation in patients presenting with lower extremity ischemia. J Vasc Surg. 2007;45:55–59 [DOI] [PubMed] [Google Scholar]
- 56.Behrendt CA, Sigvant B, Szeberin Z, Beiles B, Eldrup N, Thomson IA, Venermo M, Altreuther M, Menyhei G, Nordanstig J, Clarke M, Rieß HC, Björck M, Debus ES. International variations in amputation practice: A vascunet report. Eur J Vasc Endovasc Surg. 2018;56:391–399 [DOI] [PubMed] [Google Scholar]
- 57.Goodney PP, Likosky DS, Cronenwett JL, Vascular Study Group of Northern New E. Predicting ambulation status one year after lower extremity bypass. J Vasc Surg. 2009;49:1431–1439 e1431 [DOI] [PubMed] [Google Scholar]
- 58.American Cancer Society. Cancer facts & statistics. 2019;2019 [Google Scholar]
- 59.Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the medicare population. Vasc Med. 2008;13:209–215 [DOI] [PubMed] [Google Scholar]
- 60.Jaff MR, Cahill KE, Yu AP, Birnbaum HG, Engelhart LM. Clinical outcomes and medical care costs among medicare beneficiaries receiving therapy for peripheral arterial disease. Ann Vasc Surg. 2010;24:577–587 [DOI] [PubMed] [Google Scholar]
- 61.Huang ES, Basu A, O’Grady M, Capretta JC. Projecting the future diabetes population size and related costs for the u.S. Diabetes Care. 2009;32:2225–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.American Diabetes Association. Preventive foot care in people with diabetes. Diabetes Care. 2000;23 Suppl 1:S55–56 [PubMed] [Google Scholar]
- 63.Boulton AJM, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, Lavery LA, Lemaster JW, Mills JL Sr., Mueller MJ, Sheehan P, Wukich DK, American Diabetes A, American Association of Clinical E. Comprehensive foot examination and risk assessment: A report of the task force of the foot care interest group of the american diabetes association, with endorsement by the american association of clinical endocrinologists. Diabetes care. 2008;31:1679–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (ukpds 35): Prospective observational study. BMJ (Clinical research ed.). 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Committee for Quality Assurance. Comprehensive diabetes care. 2019;2019 [Google Scholar]
- 66.American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14–80 [DOI] [PubMed] [Google Scholar]
- 67.Smith JJ. Ncqa/hedis guidelines for diabetes. Manag Care. 2001;10:3–5 [PubMed] [Google Scholar]
- 68.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 aha/acc guideline on the management of patients with lower extremity peripheral artery disease: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2017;135:e726–e779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goodney P, Dzebisashvili N, Goodman D, Bronner K Variation in the care of surgical conditions: A dartmouth atlas of health care series 2014. [PubMed]
- 70.Leap program (lower extremity amputation prevention). Medicine and Health, Rhode Island. 2004;81:359–360 [PubMed] [Google Scholar]
- 71.Bruckner M, Mangan M, Godin S, Pogach L. Project leap of new jersey: Lower extremity amputation prevention in persons with type 2 diabetes. Am J Manag Care. 1999;5:609–616 [PubMed] [Google Scholar]
- 72.Mayfield JA, Reiber GE, Sanders LJ, Janisse D, Pogach LM. Preventive foot care in diabetes. Diabetes Care. 2004;27 Suppl 1:S63–64 [DOI] [PubMed] [Google Scholar]
- 73.Mayfield JA, Reiber GE, Nelson RG, Greene T. Do foot examinations reduce the risk of diabetic amputation? J Fam Pract. 2000;49:499–504 [PubMed] [Google Scholar]
- 74.Mayfield JA, Reiber GE, Maynard C, Czerniecki JM, Caps MT, Sangeorzan BJ. Trends in lower limb amputation in the veterans health administration, 1989–1998. J Rehabil Res Dev. 2000;37:23–30 [PubMed] [Google Scholar]
- 75.Diabetes | healthy people 2020. 2019;2019 [Google Scholar]
- 76.Czupryniak L Guidelines for the management of type 2 diabetes: Is ada and easd consensus more clinically relevant than the idf recommendations? Diabetes Res Clin Pract. 2009;86 Suppl 1:S22–25 [DOI] [PubMed] [Google Scholar]


